This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New method provides molecular insight into protein-protein interactions
Proteins are building blocks of our bodies, but they do not work solo. They form partners to facilitate in different biological processes that keep us going. However, analyzing how proteins interact at a molecular level can be challenging. Now, a research team from Japan reveals the secrets behind these "protein partnerships."
In a study published recently in Protein Science, researchers from Tokyo Medical and Dental University (TMDU) have developed a novel method by combining two techniques in a way that allows the identification of the site of interaction on two protein molecules.
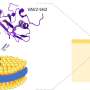
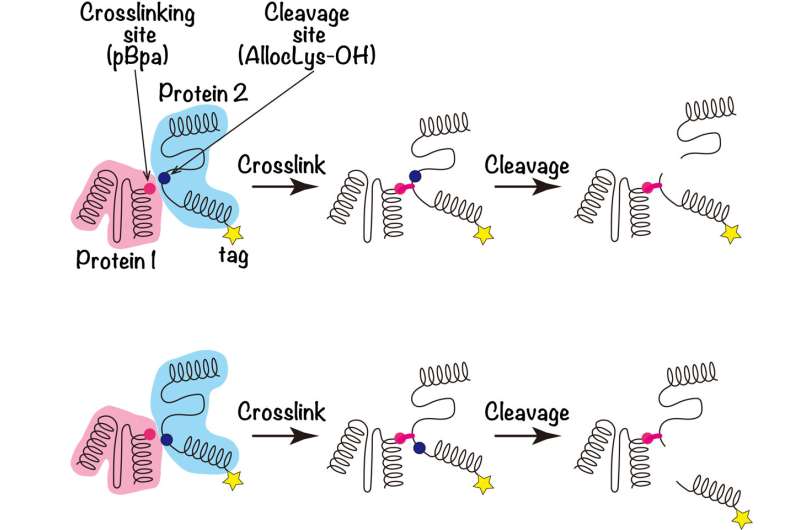
A protein consists of a chain of molecules called amino acids that then form a three-dimensional structure. An amino acid known as pBpa, which would not naturally occur anywhere else in the protein, can be introduced into a protein of interest using genetic code expansion (GCE).
Irradiation with light will then cause two interacting proteins to cross-link and stick together. However, while the site of the pBpa insertion is known, narrowing down the interacting region on the second protein is very difficult.
To solve this, the team introduced a cleavage site, "AllocLys-OH," at a known location on the second protein using GCE. "Cleaving the crosslinked proteins at this site then provides information as to which side of the AllocLys-OH site the binding interface is located at," explains first author Kazue Terasawa.
"One end of each protein is tagged in a detectable way. If the cleavage experiment separates these tags, then the cleavage site is closer to the tagged end of the protein than the crosslinked interaction site." By performing a series of these experiments with the AllocLys-OH cleavage site in different positions, the location of the binding interface on the second protein can be narrowed down.
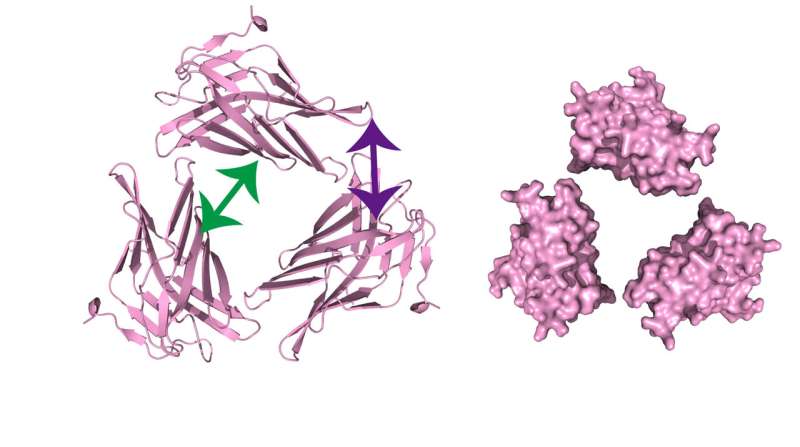
They went on to validate this technique by examining the binding site of a protein called LAMP2A. This is a major component of the membrane around a cellular structure called a lysosome and is a receptor for a process known as chaperone-mediated autophagy, which is key for breaking down proteins within a cell as part of normal cellular function. Individual molecules of LAMP2A are known to bind together in a homophilic interaction, but the details of this interaction were not fully known.
"After a series of crosslinking and cleavage experiments, we were able to clarify the geometry of the LAMP2A homophilic interaction. Based on the protein's known three-dimensional structure, the locations of the interaction sites we discovered indicate that LAMP2A must form a trimeric, or higher oligomeric, structure," explains senior author Miki Hara-Yokoyama.
This novel method is a major breakthrough for researchers studying protein–protein interactions. It allows protein-binding interfaces to be characterized, revealing complex protein geometries. It is also applicable to all mammalian cells, and therefore, it may be useful for therapeutic applications.
More information: Kazue Terasawa et al, Site‐specific photo‐crosslinking/cleavage for protein–protein interface identification reveals oligomeric assembly of lysosomal‐associated membrane protein type 2A in mammalian cells, Protein Science (2023). DOI: 10.1002/pro.4823
Provided by Tokyo Medical and Dental University