A new tool to block protein-protein interactions
Inside cells, proteins constantly interact with each other to carry out different functions. For some diseases in which these functions are altered, blocking the binding between two or more proteins emerges as a possible therapeutic approach.
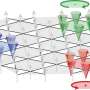
Scientists led by ICREA researcher Dr. Xavier Salvatella at IRB Barcelona have published guidelines for designing synthetic molecules that block the interaction between two proteins in the journal Nature Communications. In brief, the researchers have focused on the interactions characterized by the binding of an α-helix of one of the proteins on the surface of the other. This interaction mechanism is very common and prevalent in cell functions of therapeutic interest related to diseases such as prostate cancer.
The guidelines presented in this work allow scientists to develop molecules in a relatively straightforward manner that block (potentially) any interaction between a globular protein and an α-helix, thus offering high versatility. These synthetic molecules also show high stability, are soluble in water, and can reach the interior of the cell. Such characteristics make them ideal drug candidates.
"Our work proposes a simple way to block interactions between globular proteins mediated by α-helices and it can benefit both protein engineering and drug development efforts," explains Dr. Salvatella, head of the Molecular Biophysics Laboratory at IRB Barcelona. "It's an approach based on research performed by our lab addressing the natural interactions of certain proteins, and it proposes using this knowledge to achieve therapeutic objectives through the design of small molecules with artificial sequences," he adds.
Competition for a binding site
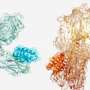
When two proteins recognize each other in the cell and interact, it is because a region on their surfaces fits, thus allowing binding. The molecules addressed in this work, like many commonly used drugs, mimic this site on the surface of one of the proteins involved in the interaction, such that they compete to bind to the site of the other protein, which is also referred to as the target protein.
Thus, if the competitor molecule is present at a higher concentration or has a greater affinity for the target protein, it will occupy all the binding sites and block any possible interaction with the original protein that the drug is mimicking. However, the size of large protein interaction interfaces makes it difficult to mimic the binding surface between them.
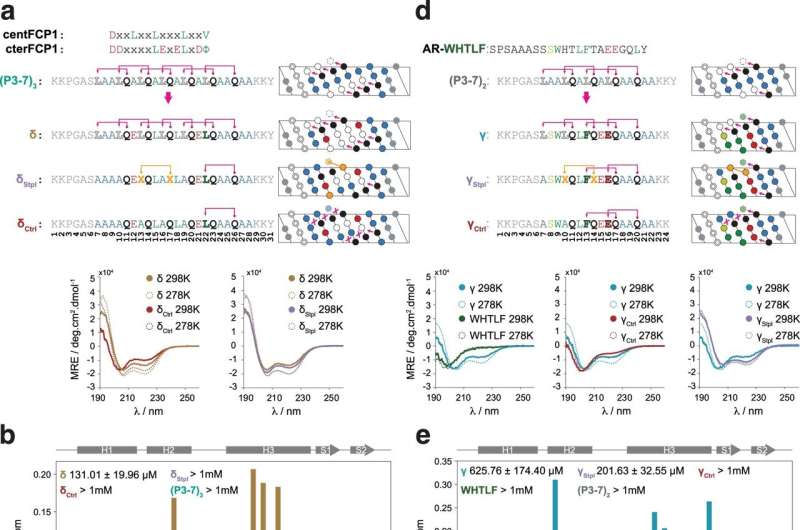
"What we propose in this work is to create molecules in the form of α-helices that offer a configurable surface to fit the target protein, and we explain how to ensure that this helix maintains a stable structure in the cellular context," explains Dr. Albert Escobedo, currently a postdoctoral researcher at the Center for Genomic Regulation (CRG), who led the work together with Dr. Salvatella at IRB Barcelona.
Describing the interactions and searching for a stable structure
The researchers have focused their efforts on detailing the characteristics that these synthetic molecules must meet to show stability and be able to perform their function of inhibiting the interaction between two proteins. In the study, they describe how several consecutive repetitions with a certain pattern of pairs of the amino acid glutamine and another hydrophobic amino acid confer stability to the helix. In contrast to other approaches with the same purpose, the exclusive use of natural amino acids and the absence of chemical modifications to stabilize the helix can enhance the biocompatibility and safety of the drugs designed using the new guidelines described.
In another study published in Nature Communications in 2019, the researchers had already observed that, for a given protein, the number of glutamine residues present in the structure condition the stability of its helix-shaped structure.
In this new study, they have confirmed that the same thing also occurs in other proteins, they explain why and use the knowledge acquired to increase the versatility of the molecules designed. Also, they propose how changes in the number of glutamine residues present in different proteins can cause different diseases.
More information: Albert Escobedo et al, A glutamine-based single α-helix scaffold to target globular proteins, Nature Communications (2022). DOI: 10.1038/s41467-022-34793-6
Journal information: Nature Communications