This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Characterizing the role of oxidized tryptophan residues in repairing damaged photosystem II protein
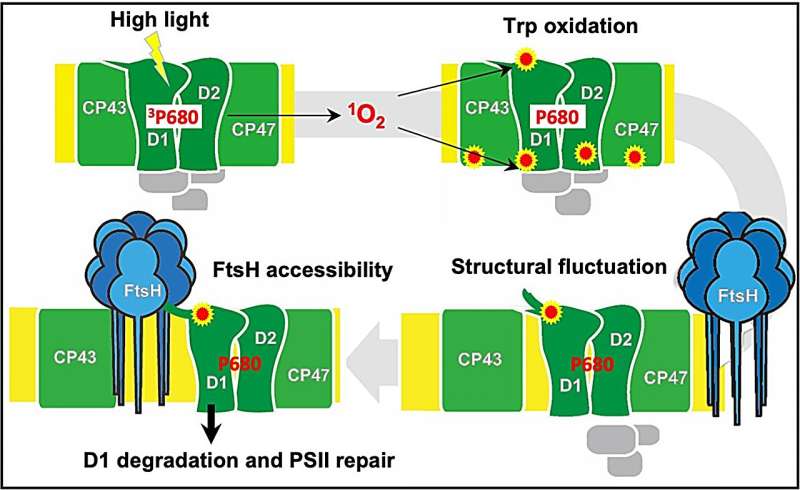
Photosynthesis refers to the fundamental biological process of the conversion of light energy into chemical energy by chlorophyll (a green pigment) containing plants. This seemingly routine process in plants sustains all the biological life and activities on Earth. The first reaction of photosynthesis occurs at a site called photosystem II (PSII), present on the thylakoid membrane in the chloroplast, where light energy is transferred to chlorophyll molecules. PSII comprises a complex group of proteins, including the D1 and D2 reaction center proteins.
Light energy plays a vital role in photosynthesis. However, a high amount of light energy can cause photo-oxidative damage in PSII, particularly to the D1 protein. The affected D1 protein is then broken down by FtsH—a protein-digesting enzyme—and a completely new D1 protein is synthesized in its place. However, how is the photo-damaged D1 protein recognized and selectively degraded by FtsH remains a mystery.
Recently, a team of researchers worked to uncover the signaling and recognition process underlying the FtsH-led D1 protein degradation event. The research team led by Professor Wataru Sakamoto from the Institute of Plant Science and Resources (IPSR), Okayama University, Japan, along with Dr. Yusuke Kato from the Faculty of Agriculture, Setsunan University, Japan, and Dr. Shin-Ichiro Ozawa from the IPSR, Okayama University, Japan, employed an innovative combination of plant biotechnology and mass-spectrometry techniques to study the photo-oxidative repair mechanism. Their research findings were published in eLife.
"The damaged photosystem-II can be understood as akin to a broken down industrial machine that needs to be repaired or replaced. I was drawn to this critical aspect of the repair/replacement process occurring during the photosynthesis, particularly the exact mechanism of this damage recognition and subsequent degradation by proteases," says Professor Wataru Sakamoto, explaining the motivation behind this study.
The team examined the recognition mechanism by tracking the presence of oxidized tryptophan amino acid residues (Trp) in the D1 protein, particularly the N-terminal Trp-14 residue. They further understood the intricate details of the D1 protein recognition by FtsH by studying the oxidative post-translational modification (OPTM) of Trp-14 residues in a variety of modified mutant species of Arabidopsis (a model plant species) and Chlamydomonas (green algae).
The researchers also used molecular dynamics (a computer-based simulation experiment) to study the functional aspects of the Trp-14 oxidation.
They observed that in addition to critically damaging the D1 protein, excess light oxidized 2 Trp residues in D1 of Arabidopsis and 4 Trp residues in Chlamydomonas, via OPTM, which marks the D1 protein for degradation by FtsH. Moreover, Arabidopsis mutant species lacking FtsH had more oxidative Trp residues in D1 and the replacement of Trp-14 to phenylalanine (Phe) in D1 of Chlamydomonas accelerated the degradation of D1 by FtsH.
"This study reveals that oxidative modification of specific amino acids can help in recognizing a damaged protein and lead to the ensuing repair mechanism. Understanding the functional aspects that sustain photosynthesis is important for our global environment, and improving the efficiency of photosynthesis processes may contribute to enhanced tolerant varieties of crops in the future," concludes Dr. Sakamoto.
More information: Yusuke Kato et al, Characterization of tryptophan oxidation affecting D1 degradation by FtsH in the photosystem II quality control of chloroplasts, eLife (2023). DOI: 10.7554/eLife.88822
Journal information: eLife
Provided by Okayama University