This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Fluorine catch-and-attach process could boost drug efficiency
When it comes to chemical reactions, fluorine has a reputation as a 'magic bullet atom' for its ability to increase a drug's absorption and prolong its lifetime. However, traditional methods of adding it to compounds entail expensive materials and can be difficult to pull off.
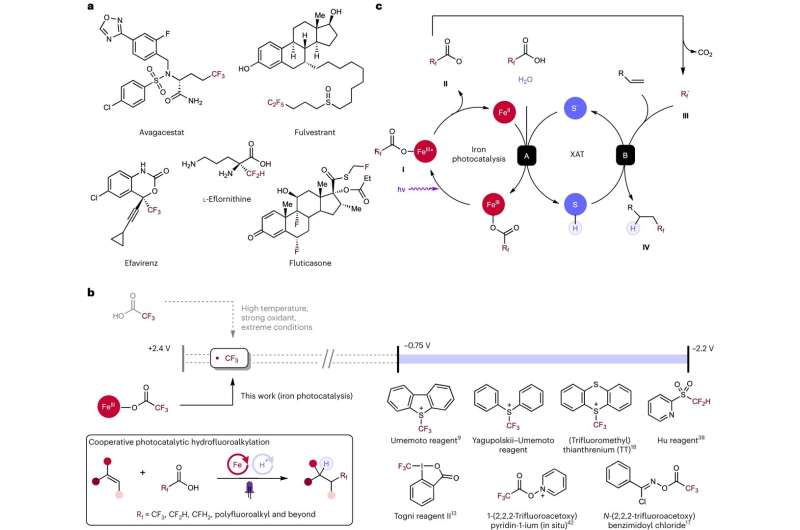
Rice University scientists developed a reliable and cost-effective process of adding fluorine to molecules for increased pharmaceutical drug efficiency using an iron and sulfur reaction. According to a study published in Nature Chemistry, the light-activated reaction can be used both to free up fluorine from carboxylic acids and to incorporate it into alkenes ⎯ common building blocks for pharmaceuticals and other chemical products. The chemical pathway developed by Rice researchers spells a new formula for easier, cheaper and more effective fluorination.
"The secret for many important drugs, from Prozac to Lipitor, is an atom called fluorine," said Julian West, the Norman Hackerman-Welch Young Investigator and assistant professor of chemistry. "When attached to drug molecules, this little atom can make the treatments act faster for longer and with fewer side effects. But it's hard to attach fluorine when making drugs. Existing processes often require chemicals that are very expensive, dangerously reactive and that just don't work very well."
The West research group wondered if it could find a way to add fluorine to molecules through a cheap, efficient, safe process.
"This was a big, pie-in-the-sky idea when we started our research group in 2019, but we were feeling pretty confident we could do it after discovering that iron and sulfur can work together to create a kind of reaction called decarboxylation," West said.
Unlike the noble metal starting materials used to synthesize molecules for a variety of industries, more common elements like iron and sulfur are easily accessed, inexpensive, and abundant around the world. Earlier this year, the West research group found that when it shines light on iron and sulfur, they interact like rowdy bar patrons that erupt into a melee with enough energy to break apart carboxylic acid molecules, freeing up fluorine atoms for use elsewhere.
"Carboxylic acids are everywhere; they are cheap, available, and hardy," West said. "The problem is that their very stability makes them difficult to break into useful parts. If iron and sulfur could decarboxylate these common materials, making their fluorine atoms available, then we would be off to the races."
Once the researchers determined how to free up the fluorine from these stable compounds, they next had to figure out how to reattach it in an effective and safe manner. Rice doctoral alum Yen-Chu Lu and chemistry graduate student Kang-Jie (Harry) Bian figured out that fluorinated carboxylic acids could react with alkenes, another common part of drug molecules.
"Alkenes are most commonly used to help put the drugs together because they can be transformed into many different things, like a foundation that more complex parts can be built on," West said. "Yen-Chu and Harry determined the same iron and sulfur combination that cracked the carboxylic acid could facilitate attachment of the newly revealed fluorine fragment to alkenes."
Not only is the new strategy fast, simple and safe, but it also seems applicable in a wide variety of uses.
"If you want to add a different fluorine piece, just put in a different carboxylic acid," West said.
Lu and Bian worked with Rice graduate student Shih-Chieh Kao and undergraduates Xiaowei Chen and David Nemoto Jr. to stress-test the strategy.
"They really pushed this approach to its limits and haven't yet discovered its boundaries," West said. "The team did an amazing job, and I'm super lucky to have such creative coworkers in our group."
More information: Bian, KJ. et al, Photocatalytic hydrofluoroalkylation of alkenes with carboxylic acids, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01365-0. www.nature.com/articles/s41557-023-01365-0
Journal information: Nature Chemistry
Provided by Rice University