This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Researchers discover new superacid
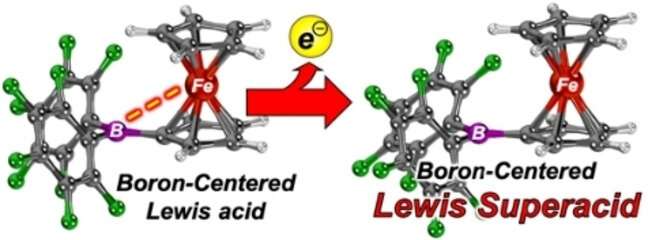
Researchers at Paderborn University have succeeded in producing very special catalysts, known as "Lewis superacids," which can be used to break strong chemical bonds and speed up reactions. The production of these substances has, until now, proven extremely difficult.
The chemists' discovery enables non-biodegradable fluorinated hydrocarbons, similar to Teflon, and possibly even climate-damaging greenhouse gases, such as sulfur hexafluoride, to be converted back into sustainable chemicals. The researchers have now published their results in Angewandte Chemie.
"Lewis acids" are compounds that add electron pairs. Because of this ability, they are often used to speed up chemical reactions. Lewis superacids are stronger than antimony pentafluoride—the strongest Lewis acid—and can break even the toughest bonds. Professor Jan Paradies from the Department of Chemistry at Paderborn University explains, "For strong bonds, you need highly reactive reagents, i.e. substances that are extremely reactive."
The new catalyst can split, for example, carbon-fluorine or sulfur-fluorine bonds, which are particularly robust. "Intrinsically, these Lewis superacids are incredibly reactive, which makes them difficult to produce and use. Using a trick, we managed to produce such molecules and use them in catalytic reactions. This makes it possible to, for example, activate and further convert virtually inert, i.e. less reactive, carbon-fluorine or sulfur-fluorine bonds," says Paradies.
The researchers in the Department of Chemistry have joined forces in the Center for Sustainable Systems Design (CSSD), which bundles interdisciplinary basic research in the field of sustainability at Paderborn University. The complementary scientific interests of the groups involved form the nucleus for new approaches in the development of sustainable systems.
The center wants to leverage this capacity to contribute to the sustainable use of resources. This and related topics are also covered in Paderborn within the framework of its "Sustainable Chemistry' master's program.
Paradies says, "We're thereby placing a clear emphasis on sustainability not only in research, but also in teaching."
More information: Laura Köring et al, Boron‐Centered Lewis Superacid through Redox‐Active Ligands: Application in C−F and S−F Bond Activation, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202216959
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Universität Paderborn