Activation of carbon-fluorine bonds via cooperation of a photocatalyst and tin
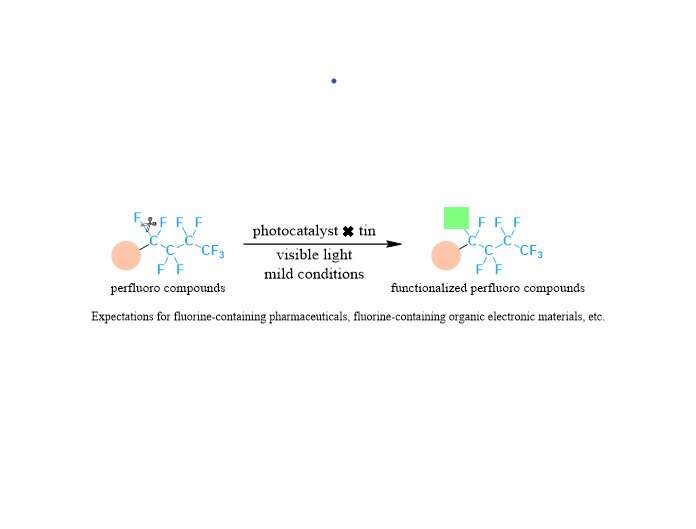
Fluorinated compounds are an important group of compounds that are widely used in pharmaceuticals, agricultural chemicals, functional resins, and organic electronic materials. In particular, perfluorinated compounds with multiple carbon-fluorine bonds are attracting attention because of their high thermal and chemical stability and various excellent properties such as water and oil repellency and chemical resistance.
"C-F bonds are extremely strong; hence, their transformation under mild conditions is difficult, and the selective activation of a specific C-F bond from among multiple C-F bonds in perfluorinated compounds has not been achieved," explains Prof. Makoto Yasuda, corresponding author of the study.
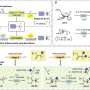
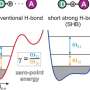
The research team led by Prof. Makoto Yasuda has discovered that a site-selective C-F bond transformation to valuable functional groups proceeds via a photocatalyst and organotin compounds under visible light irradiation. Experimental and theoretical results revealed the importance of the cooperation of a photocatalyst and organotin compounds in this transformation.
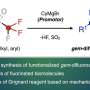
In this research, site-selective C-F bond transformation to valuable allylic groups has been accomplished by using a photocatalyst and organotin compounds under safe and common visible light irradiation. The establishment of the methodology to activate strong carbon-fluorine bonds under such mild conditions is the key to achieving the targeted transformation of perfluorinated compounds at specific sites.
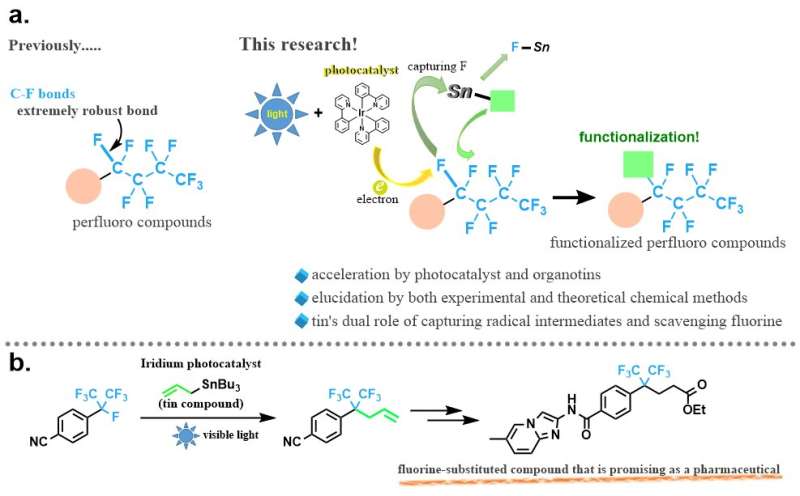
"We have attempted to elucidate this reaction mechanism using both experimental and theoretical chemical methods and have found that the cooperative action of the photocatalyst and organotin compound plays a very important role in the progression of the reaction. In particular, it is noteworthy that the organotin compound plays the dual role of capturing unstable radical intermediates and scavenging fluorine as a Lewis acid, which is a very significant finding for future research on carbon-fluorine bond conversion reactions," explains Prof. Makoto Yasuda. Furthermore, by using this method, they have succeeded in synthesizing fluorine-substituted analogues of a compound that show promise for pharmaceutical applications.
"Fluorine is an important element in pharmaceuticals, and many small-molecule drugs contain fluorine atoms. It is expected that the field of fluorine-containing drugs will continue to grow. As a result of this research, high value-added perfluorinated compounds, which were impossible to synthesize in the past, can now be synthesized in a simple and short process, which is expected to lead to the expansion of the library of seed compounds for fluorine-containing drug discovery," says Prof. Makoto Yasuda.
The article, "Photoredox-catalyzed C-F bond allylation of perfluoroalkylarenes at the benzylic position" was published in the Journal of the American Chemical Society.
More information: "Photoredox-catalyzed C-F bond allylation of perfluoroalkylarenes at the benzylic position" Journal of the American Chemical Society (2021). DOI: 10.1021/jacs.1c03760
Journal information: Journal of the American Chemical Society
Provided by Osaka University