This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemical process makes peptide acquire structure similar to amyloid plaques found in neurodegenerative diseases
Peptides are biomolecules formed when two or more amino acids that perform key functions in the human organism, such as hormones, neurotransmitters, painkillers and antibiotics, bind together. For this reason, they are much studied and used by the pharmaceutical industry, for example.
A study conducted by scientists in the Department of Biophysics at the Federal University of São Paulo's Medical School (EPM-UNIFESP) in Brazil identified significant changes in the physicochemical properties of peptides during a spontaneous process of chemical change called pyroglutamination.
Pyroglutamination is a modification resulting from spontaneous conversion of glutamine to pyroglutamic acid, with a significant impact on the physical and chemical properties of peptides. It is a well-known but frequently overlooked part of peptide synthesis, and rarely explored in proteomics.
The researchers who conducted the study stress that it can occur rapidly and accelerates as temperature rises, underscoring the need for caution during laboratory experiments to prevent glutamine cyclization. It is especially important in conditions that mimic physiological environments where temperatures are in the range of 37° C, the normal temperature of a healthy human organism.
The discovery has implications for laboratory research and opens up new prospects for the study of neurodegenerative diseases such as Alzheimer's and Parkinson's, since after chemical modification the molecule acquires an amyloidal structure, which favors aggregation of molecules, forming plaques like those believed to cause the diseases in question.
An article on the study is published in Biochemistry.
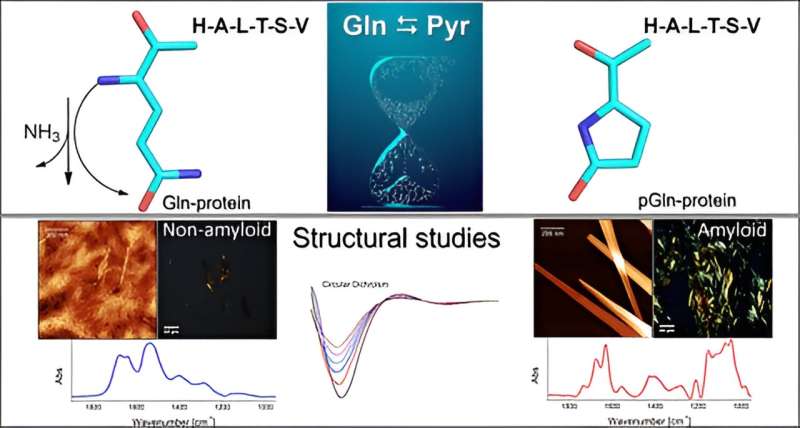
The group conducted in vitro experiments to investigate the mechanism whereby the amino acid glutamine (Gln) becomes pyroglutamic acid (Pyr) in the presence of a peptide or protein sequence at the N-terminal extremity. This process occurs through deamidation, a reaction that eliminates ammonia (NH3). Pyr (also called pyroglutamate) is a cyclic amino acid formed as a result of dehydration of glutamate. All proteins consist of multiple amino acids joined together by peptide bonds, with variations in the number and sequence of amino acids.
"The result can serve as a model for many researchers who work with peptides. We arrived at two key findings. We returned to an old topic, which is how glutamine breaks down into pyroglutamic acid, but we introduced a warning about the importance of analyzing the sequence. The second point was that after conversion of the peptide, its characteristics change and it tends to stick to membranes."
"The presence of pyroglutamic acid favors the formation of amyloidogenic aggregates, similar to the conglomerates typically found in cases of neurodegenerative disease. These amyloid plaques are formed in the brain and interrupt the flow of neurons," said Clovis Ryuichi Nakaie, last author of the article.
Stages of the research
The model peptide sequence (QHALTSV-NH2) used in the study originated in the Ph.D. research of Mariana Machado Leiva Ferreira, first author of the article, while she was looking for a synthesis of some two dozen peptides present in the sequences of five G-protein coupled receptors (GPCRs) that varied in size up to about 20 amino acids. GPCRs capture a wide array of extracellular signals (ranging from photons to ions, proteins, neurotransmitters and hormones) and activate signaling pathways inside cells.
One of the peptides synthesized by Ferreira stood out for its low yield and was the only one with glutamine at the amine extremity. "After the first attempt at synthesis with a very low yield, we varied several parameters to increase production of the peptide, including changes to the synthetic part and to the purification process, but unfortunately it always partially degraded," she said.
When the group tested solutions frequently used in proteomic experiments, they found that glutamine conversion to pyroglutamic acid occurred in all of them as a function of time, in accordance with typical first-order kinetics, where the rate of conversion was proportional to the time taken by the reaction. They then decided not to agitate the solution so that the conversation rate could be inferred. For example, they estimated that after five hours at least 10% of the glutamine probably converted into pyroglutamic acid.
A minor structural change triggered when the native peptide was pyroglutaminated at the N-terminal extremity was sufficient to change the molecule's physicochemical behavior.
"Because it's cyclical and has one less positive charge, the peptide Pyr should be more hydrophobic than the native molecule, and we therefore expected the analog to interact with membrane-mimetic systems. What we didn't foresee was that the analog would entail the formation of amyloid structures like those seen in neurodegenerative diseases. We didn't study any of these, but our results point in that direction," Emerson Rodrigo da Silva, penultimate author of the article, told Agência FAPESP. Silva and Nakaie are the corresponding authors.
Nakaie stressed the importance of post-translational changes in the organism involving the polypeptide chain. They play a role in the functional diversity of proteins and enable adaptation of a sequence of amino acids encoded by a gene to perform various regulatory functions.
"In this context, time as a factor will always correlate with the occurrence of changes, regardless of their speed or their location in our organism. This recalls the idea of the biological clock and is the reason why we suggested putting an hourglass on the cover of the journal to symbolize the spontaneous conversion of Gln into Pyr," Nakaie said.
He has been a professor at EPM-UNIFESP for 45 years and stressed the groundbreaking work done by the group in the Department of Biophysics. In particular, he noted, they introduced the synthesis and biochemistry of peptides and amino acid derivatives to Brazil.
"Our findings will undoubtedly pave the way for further studies. After completing the work of which Mariana Ferreira's Ph.D. research was part, we also want to go on with this research line," he said.
More information: Mariana M. L. Ferreira et al, Pyroglutamination-Induced Changes in the Physicochemical Features of a CXCR4 Chemokine Peptide: Kinetic and Structural Analysis, Biochemistry (2023). DOI: 10.1021/acs.biochem.3c00124
Journal information: Biochemistry
Provided by FAPESP