November 24, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Examining the biodistribution and function of polymer-DNA origami nanostructures
The capacity to regulate the biodistribution of therapeutics is a highly desired feature that can limit the side effects of many drugs. In a new study in Scientific Reports, Noah Joseph, and a team of biotechnology and nanoscience scientists in Israel, describe a nanoscale agent developed from a coupled polymer-DNA origami hybrid capable of exhibiting stability in serum and slow diffusion through tissues.
By coupling to fragments of polyethylene glycol through polyamine electrostatic interactions, the team noted marked stability of the agents in vivo, where more than 90% of the constituents maintained structural integrity for five days after subcutaneous injection.
The findings highlight the polymer-DNA hybrid nanostructures as viable pharmacological agents that can enter mainstream technologies, including their use as monoclonal antibodies for drug activity.
DNA origami therapeutics
Many drugs, including small molecules and biologicals function systematically without the innate capacity for distribution and function. This is the central driving force of adverse effects and a major component of drug impairment for many new drugs in clinical trials and clinical use.
While great efforts were made in the past decades to achieve drug activity regulation, at present the approved drugs only represent a small fraction of the true potential of the therapeutic mechanisms of drugs.
Monoclonal antibodies are a mainstream and well-proven pharmaceutical method that exemplifies this challenge. The monoclonal drugs have enabled breakthrough treatments in diseases that have hitherto been considered nearly untreatable in oncology, immunology and inflammatory diseases. Scaffolded DNA origami is a method to develop DNA nanostructures and facilitate the precise spatial regulation and functionality at the sub-nm scale.
A new strategy for DNA therapeutics
The unique properties are suited across a variety of research fields, to mark them as next-generation therapeutic and diagnostic agents. A variety of DNA origami functionalization methods can achieve higher functional complexity when compared with monoclonal antibodies.
In this novel strategy presented by Joseph and colleagues, the team facilitated the spatial regulation of drug activity by coupling polymer-DNA origami hybrid nanoscale agents. These designs can be adapted across several target proteins for a variety of pathologies of wide-ranging therapeutic functionality.
In this work, Joseph and colleagues presented a strategy to deliver therapeutic drug constituents based on coupled polymer-DNA origami hybrid nanoscale compounds. By following the usual, kinetic and stability characterization studies of several DNA origami constructs in vivo, the scientists selected an optimal DNA nanostructure as a proof-of-principle for therapeutic applications with highly potent anti-inflammatory effects in a mouse model and in human Tumor Necrosis Factor alpha.
The experiments
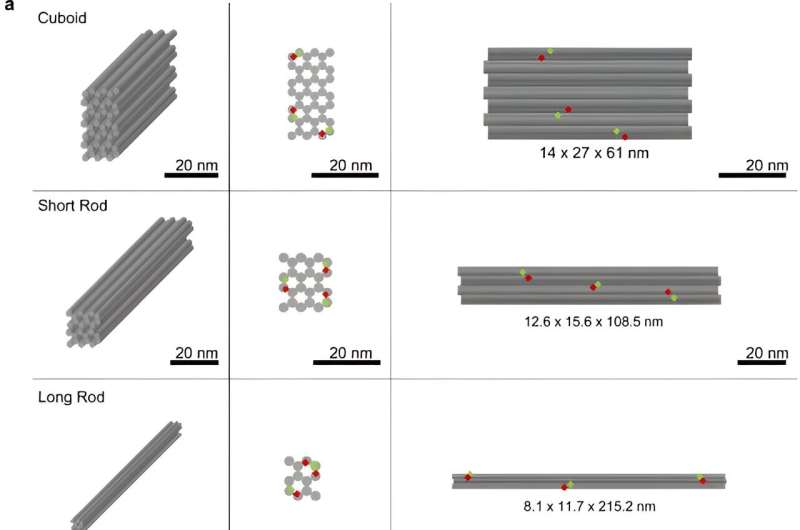
To begin the proof-of-feasibility study, the research team chose three different DNA origami nanostructures of similar mass and analyzed them with gel electrophoresis to determine the bulk quality. They used transmission electron microscopy before and after coating the DNA nanostructures with polyethylene glycosylate-polylysine through amine and phosphate interactions to increase the mass of DNA and increase their attachment to polyethylene glycosylate and ensure the stability of the DNA origami nanostructures.
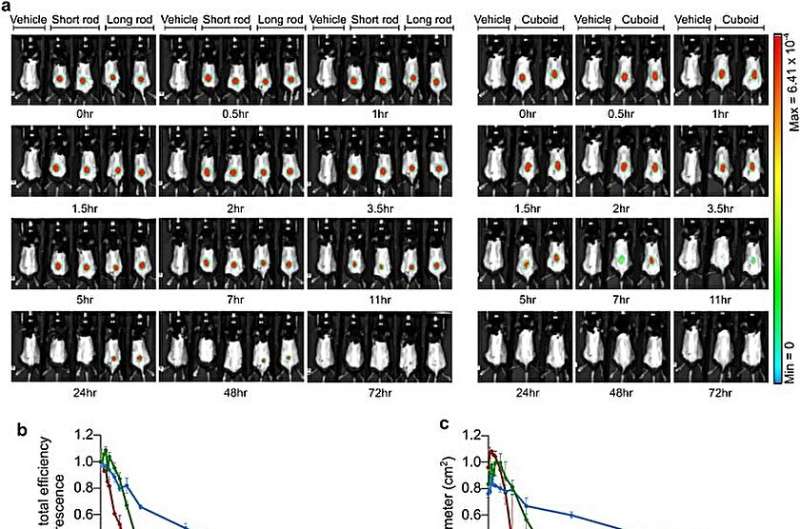
Drugs with in vivo stability are suited for distribution and the team explored this by performing live imaging of mice treated with the polymer-coated nanostructures administered subcutaneously into knee joints or intraperitoneally into mice.
While the long rod showed extended diffusion through time, it was possible to combine slower diffusion with greater stability subcutaneously. The scientists explored the kinetics and the in vivo stability of the findings to select the polymer count rod nanostructures as efficient constituents for druggable experiments.
Therapeutic effects of the DNA origami nanostructures
The scientists studied the redesigned long rod nanostructures to represent the human tumor necrosis factor alpha aptamers and anchored them uniformly across the surface structures. Joseph and colleagues analyzed the functionalization of long rod DNA origami structures by using agarose gel electrophoresis, transmission electron microscopy, and atomic force microscopy.
The team examined the stability of the constituents in human serum for 10 days and identified its structural integrity for biodistribution and in vivo studies.
Outlook
In this way, Noah Joseph and the research team describe the in vivo kinetics of three DNA origami nanostructures of different shapes stabilized by the polyethylene glycol-polylysine polymer. The scientists chose the optimal candidate and functionalized the long rod nanostructures by attaching human tumor necrosis factor alpha aptamers to target the human tumor necrosis factor alpha protein.
The research team describes the therapeutic potential of the functionalized co-polymer DNA origami nanostructures to function across complex biological environments. The combined findings highlight the influence of the DNA nanostructures as a significant therapeutic agent for precision medicine and functionality of therapeutic agents.
More information: Noah Joseph et al, Biodistribution and function of coupled polymer-DNA origami nanostructures, Scientific Reports (2023). DOI: 10.1038/s41598-023-46351-1
Journal information: Scientific Reports , Science Advances
© 2023 Science X Network