This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Improving nanotherapeutic vaccine delivery
Northwestern Medicine scientists have developed a more effective way of creating nanotherapeutic vaccines and medicines, according to a new study published in ACS Nano.
"Over the last decade, spherical nucleic acid, or SNA, technology has emerged as a broad therapeutic platform for a wide variety of diseases, including cancer and other illnesses," said Chad Mirkin, Ph.D., professor of Medicine in the Division of Hematology and Oncology, the George B. Rathmann Professor of Chemistry at Northwestern's Weinberg College of Arts and Sciences, and director of the International Institute for Nanotechnology, who was the lead author of the study.
In the Mirkin laboratory, investigators have harnessed this SNA technology in their work to design precision nanomedicines for use in gene regulation and in cancer immunotherapy with limited unwanted side effects through a systematic development process known as rational vaccinology.
"In the development of vaccines, historically, very little attention has been paid to vaccine structure," said Mirkin, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. "All of the emphasis has been on the components. The premise of rational vaccinology is that, while components are critical, structure is equally important. How you present vaccine components within a modular nanoscale architecture can have a dramatic impact on vaccine efficacy, whether it's treating infectious disease or cancer."
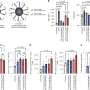
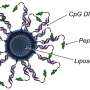
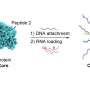
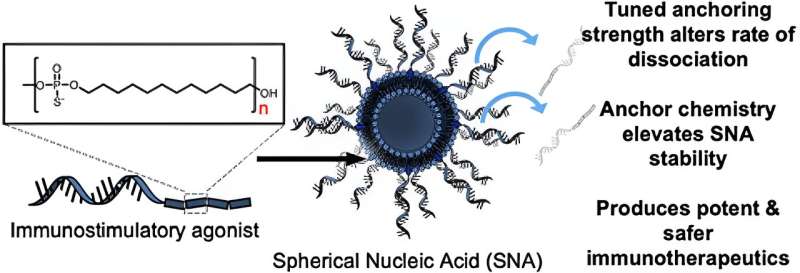
In the study, investigators first tested the effects of using different chemical anchoring groups to attach the oligonucleotides—short strands of DNA or RNA—to the surface of the liposomes to prepare SNAs. They found that when increasingly hydrophobic dodecane-based anchoring groups were used, the stability of the nanostructure was significantly improved. When introduced to bone marrow-derived dendritic cells from mice, these more stable SNA constructs exhibited improved cellular uptake compared to the other versions of SNAs that were prepared using other types of anchoring groups, with different chemistries.
"We discovered a way to anchor the oligonucleotides to the surface of the particle that changes the overall stability of the SNA construct, which is critical," said Jasper Dittmar, a Ph.D. student in the Mirkin laboratory and a co-author of the study. "The beauty of the SNA architecture is that it's recognized by almost all cell types, immune cells included, and rapidly internalized. You get the vaccine to enter the cells that matter at the stoichiometry you'd like, with the desired number of antigens and adjuvant molecules."
Scientists in the Mirkin lab then loaded the SNA vaccine with OVA1 (a model peptide derived from egg protein often used in vaccine development) and administered that to mice with lymphoma. The OVA1 SNA-treated mice not only had a greater number of polyfunctional T-cells (which are considered potent against chronic infections and tumors), they also showed a 21-fold reduction in tumor volumes compared to saline-treated mice, according to the study.
To assess the inflammatory side effects of the vaccine, investigators then studied the SNA to see if it activated excessive immune responses in mice. Mice given the treatment did not produce a cytokine storm, a sometimes-fatal side effect of immunotherapies.
Because cytokine storms are associated with severe cases of COVID-19, Mirkin and his research team also created an SNA vaccine where the OVA1 peptide was swapped out for a peptide from the virus that causes COVID-19 (CoV peptide) and administered it to human cells and ultimately mice. The investigators found that the vaccine enhanced antigen-specific, anti-COVID immune responses with minimal adverse side effects.
"Taken together, the results of this study lay a foundation for a new way of developing and delivering vaccines and other precision treatments, regardless of the target disease," said Michael Evangelopoulos, a Ph.D. student in the Mirkin lab and a co-author of the study.
The findings also highlight the importance of vaccine construction, Mirkin said.
"Structure matters," Mirkin said. "In a field where we've spent very little time focused on the structure of vaccines, we have may have been missing the forest for the trees. It's a combined understanding of the components and the structural presentation that leads to an efficacious medicine or not."
Moving forward, the Mirkin group will continue to devise different configurations of SNA vaccines to assess which are the most effective, he said.
"We are spending a lot of time using the SNA platform to figure out the structures that are the most efficacious, and then trying to figure out why that is, what works and then also why it works," Mirkin said. "We think that by doing that, we'll be able to create a whole new generation of medicines based upon this concept of rational vaccinology."
More information: Jasper W. Dittmar et al, Tuning DNA Dissociation from Spherical Nucleic Acids for Enhanced Immunostimulation, ACS Nano (2023). DOI: 10.1021/acsnano.3c04333
Journal information: ACS Nano
Provided by Northwestern University