This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Understanding drivers of egg cell development
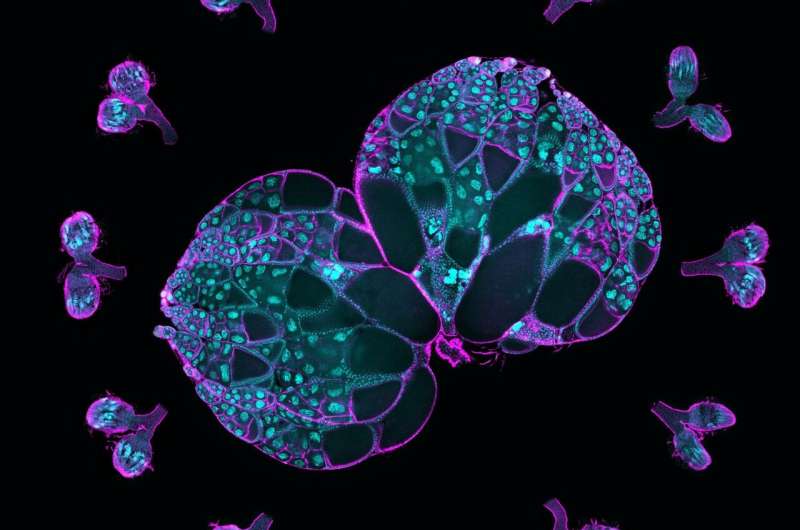
Northwestern Medicine scientists have identified how cytoskeletal proteins contribute to the growth of developing eggs in fruit flies, findings that further the field's understanding of how egg cells form and differentiate themselves from other sister cells, according to a new study published in the Proceedings of the National Academy of Sciences.
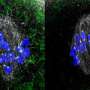
Oocytes, or developing eggs, are formed from a collection of sister cells inside an ovary when one cell differentiates itself as an oocyte and the others become "nurse" cells supplying it with "building materials," the mRNAs, proteins and organelles needed to grow. The nurse cells feed the oocyte and then die off as part of the development process, said Wen Lu, Ph.D., research assistant professor of Cell and Developmental Biology and first author of the study.
"We call this 'winners take all,' and these interconnected cellular structures are highly conserved; you can see this happen all the way from fruit flies, our model system, to mammals, including human beings," Lu said.
The mechanisms behind this process, however, were not well understood and prompted further exploration by the laboratory of Vladimir Gelfand, Ph.D., the Leslie B. Arey Professor of Cell, Molecular, and Anatomical Sciences and senior author of the study.
"Essentially these oocytes are parasites," said Gelfand, who is also a professor of Cell and Developmental Biology. "They are not making proteins; everything is done in the nurse cells and pumped into the oocyte along microtubules, these tiny cargo tracks, by a molecular motor cytoplasmic dynein."
In the current study, scientists cultured ovaries from fruit flies, which share roughly 75% of their DNA with humans and undergo similar cellular development processes.
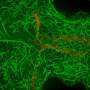
Then, investigators used live imaging to track proteins within the oocyte and nurse cells. They observed that an oocyte generated a larger number of microtubules, intercellular "highways" used to access and transport proteins and other resources from nurse cells.
In humans, the protein XMAP215 promotes microtubule growth, while in fruit flies, this process is regulated by a genetic homolog called mini spindles (Msps).
Scientists then found that Msps mRNA (messenger RNA), which carries instructions for making Msps protein, was concentrated in the oocyte cell. Knockdown of the mRNA negatively affected microtubule development and cell growth, according to the study.
Using single-molecule fluorescence in situ hybridization, investigators found that dynein, a cytoskeletal motor protein that moves cargo along microtubules in cells, was responsible for accumulating the Msps mRNA within the oocyte.
Removing dynein decreased the amount of mRNA within the oocyte, according to the study, which arrested normal cell development.
The findings identify Msps and dynein as a dynamic duo in governing oocyte specialization and development, Gelfand said. Dynein brings more Msps mRNA into the oocyte, and that mRNA produces more Msps protein, which, in turn, creates more microtubule tracks for the dynein motor. In this way, dynein and Msps form a positive feedback loop that promotes oocyte development, Gelfand said.
"Oocyte identity is a key question of developmental biology," Gelfand said. "Knowing what is involved in defining the oocyte out of the 16 interconnected cells it is born from is important because it's the very first stage of development. It's helpful to know that microtubules and microtubule motors are a key part of that."
Future investigation will focus on the factors that determine which cells become oocytes, Gelfand said.
"We want to know the mechanism behind this," said Gelfand, who is a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. "Is the transport of mini spindles to a cell enough to define an oocyte? If we pump it into a cell that is not supposed to be an oocyte, would that be enough to convert it? Using optogenetics, we will try and tip the scales and convert the cell's fate."
More information: Wen Lu et al, The dynamic duo of microtubule polymerase Mini spindles/XMAP215 and cytoplasmic dynein is essential for maintaining Drosophila oocyte fate, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2303376120
Journal information: Proceedings of the National Academy of Sciences
Provided by Northwestern University