This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
CLAMP complex helps parasites enter human cells
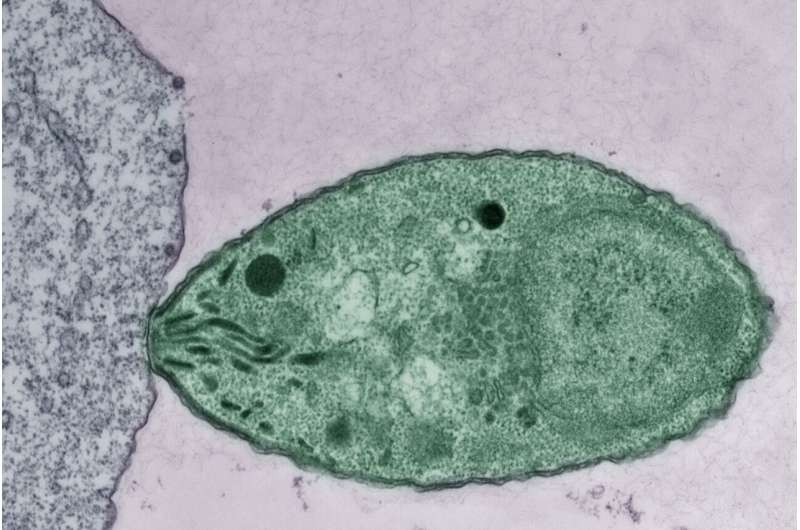
Apicomplexan parasites are a group of single-celled organisms responsible for several serious and prevalent diseases, from malaria, to a severe childhood diarrhea (cryptosporidiosis), to toxoplasmosis—a disease that endangers pregnant women and fetuses, and is the reason pregnant women are told to avoid changing cat litter.
The parasites are hard to eliminate, which is why they infect hundreds of millions of people each year. The more that researchers learn about how the parasites operate, the better equipped they will be to develop effective vaccines and drugs against them.
In the parasites' active stage, they spend most of their time replicating inside of host cells. However, sometimes they must leave in order to find new cells to invade. This time spent outside the cell is dangerous for the parasites—it is when they are most vulnerable to detection by the immune system—so they must engineer their way inside a new host cell quickly. Invasion of host cells is a crucial step in the parasite life cycle, and yet the details of how parasites achieve it have not been well understood.
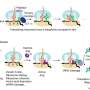
New research from Whitehead Institute Member Sebastian Lourido and colleagues, published in the EMBO Journal on October 27, identifies a complex of three proteins, CLAMP, CLIP, and SPATR, that plays a key role in helping apicomplexan parasites carry out host cell invasion. The research shows that the complex is required to trigger the release of molecules that the parasites use to enter new cells.
Before invasion, parasites store these molecules in large cargo-containing organelles called rhoptries. The CLAMP complex triggers the rhoptries to release their cargo only once a parasite is in position at a new host cell. Apicomplexan parasites do not have many rhoptries—when invading blood cells, the species that spreads malaria only has two—and so if parasites release their rhoptry contents in the wrong time or place, they will miss their shot to invade a new cell.
CLAMP, CLIP, and SPATR are not the only proteins required to trigger the discharge of rhoptries. Researchers have previously identified a few others. However, uncovering the CLAMP complex's role gives researchers a more complete picture of how the parasites invade host cells. Additionally, the CLAMP complex is an interesting possible target for drugs or vaccines: it is found in some form in all apicomplexan species, but not found in humans or other mammals.
This means that its inhibition should prevent the parasite from invading cells without disrupting any cellular machinery in the host, and that therapeutics designed to target it might be easy to adapt across parasite species. Furthermore, part of the CLAMP complex extends outside of the parasite, and so would be accessible to antibodies or other therapies.
"With vaccines, the target is often the entry protein that the pathogen uses to get into the cell, like the spike protein on the virus that causes COVID-19. What we've identified here is part of the entry machinery for apicomplexan parasites, and although this machinery is much more complex than that found in viruses, I think this could be a really interesting target for a vaccine strategy," says Lourido, who is also an associate professor of biology at the Massachusetts Institute of Technology.
Determining what the CLAMP complex does
Co-first author Saima Sidik, then a staff scientist in Lourido's lab, and colleagues first identified the protein CLAMP in a genetic screen of the apicomplexan parasite Toxoplasma gondii (T. gondii) in 2016 looking for essential genes. They found that the protein was necessary for the parasite to invade new cells, but did not know the step of invasion to which it contributed.
"It's always fun to see how the parasite comes up with unique strategies for survival within its hosts," Sidik says. "CLAMP interested us because it is similar to mammalian proteins that are involved in cell-cell interaction, but the parasite had put its own spin on that familiar biology, and parts of it were unlike anything we'd seen."
Now, Sidik and co-first author Dylan Valleau, a postdoc in Lourido's lab, have significantly fleshed out their understanding of CLAMP. They found that CLAMP forms a complex with two other proteins: SPATR, which had been identified previously but had an unknown function in invasion, and CLIP, which had not been identified previously.
The three proteins are found in micronemes, another type of cargo container that apicomplexans discharge before rhoptries. Microneme contents enable the parasites to exit a host cell, travel to another, and prepare to invade it. Rhoptry contents are also needed to complete invasion—they help the parasite enter the host cell and rewire it to inactivate anti-parasitic protections—and this is where the CLAMP complex comes in.
Without the complex, an apicomplexan parasite can still latch onto a host cell and align itself so that its tip is pressed into the host cell's membrane. However, the complex is required for the next step, in which the parasite expels the contents of its rhoptries from its tip directly into the host cell, kickstarting invasion. The researchers discovered this through experiments in T. gondii, which causes toxoplasmosis.
Determining how the CLAMP complex does it
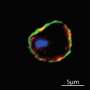
The researchers also figured out how the CLAMP complex is structured, which can provide clues to how it functions. During invasion, the complex stretches across the parasite's cell membrane, with CLAMP's tail inside of the parasite and SPATR completely outside of the parasite. The researchers speculate that the complex may relay a signal from the host cell across the parasite membrane, to let the parasite know when it is in position and ready to discharge its rhoptries. Several of their findings support this model, including that the removal of CLAMP's tail—which they propose transmits the signal inside of the parasite—prevents discharge of the rhoptries.
Learning more about the specifics of how the CLAMP complex functions will hopefully prove useful for fighting the diseases apicomplexan parasites cause. The researchers also suspect that it may provide insights into inter-cell signaling.
"CLAMP is similar to a molecule found in mammals at junctions between cells, and its tail looks like a signaling domain," Valleau says. "Figuring out how this protein allows a signal to cross the parasite cell membrane, and what other molecules it works with within the parasite, could provide new insights into cell-cell interactions, which are relevant to many aspects of biology."
More information: Dylan Valleau et al, A conserved complex of microneme proteins mediates rhoptry discharge in Toxoplasma, The EMBO Journal (2023). DOI: 10.15252/embj.2022113155
Journal information: EMBO Journal
Provided by Whitehead Institute for Biomedical Research