This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Investigating the link between iron deficiency and regulation of cell growth
Northwestern Medicine investigators have uncovered new mechanisms by which iron deficiency inhibits cell growth and proliferation in eukaryotic cells, according to findings published in Nature Cell Biology.
The study, led by Hossein Ardehali, MD, Ph.D., the Thomas D. Spies Professor of Cardiac Metabolism, has implications for improving the understanding of both the physiological and pathological states of anabolism (cell growth and proliferation), such as cancer, as well as the development of new cancer therapies.
"In the most general sense, we believe we have uncovered one of the oldest iron-sensing pathways common to eukaryotes and linked that to control of anabolism through the mTOR pathway," said Jason Shapiro, Ph.D., a student in the Medical Scientist Training Program (MSTP) and lead author of the study.
All eukaryotic cells require a minimum iron threshold to sustain anabolism, but the mechanisms that enable cells to sense the amount of iron needed to regulate anabolic processes have been poorly understood.
In a previous study from the Ardehali laboratory, scientists discovered a link between iron deficiency and anabolism through the inhibition of the mTOR (mammalian target of rapamycin) signaling pathway, which serves as the metabolic hub of the cell, according to Ardehali.
"It [mTOR] responds to a number of growth factors, including amino acids and nucleotides, and when it senses nutrients that are available in the environment, it increases protein synthesis and increases the anabolic processes inside the cell," said Ardehali, who is also professor of Medicine in the Division of Cardiology, of Pharmacology and director of the Center for Molecular Cardiology.
Building off those previous findings, Ardehali's team aimed to identify the mechanisms that inhibit the mTOR pathway in response to iron deficiency in eukaryotic cells.
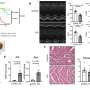
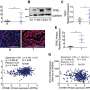
In the current study, the team analyzed different types of iron-deficient eukaryotic cells as well as livers from mice fed an iron-deficient diet. In doing so, they identified genome-wide changes to histone methylation—a regulatory process that helps turn genes on and off—within the cells.
Specifically, they found that KDM3B, an iron-binding enzyme, acts as an iron sensor and inhibits mTOR activity through histone demethylation. Iron deficiency inactivates KDM3B, resulting in suppression of LAT3, an amino acid transporter, and RAPTOR, a conserved protein in the mTOR pathway.
"We think that there are two pathways that are responsible for the response to iron deficiency. One is through reducing one component of the mTOR complex, which is RAPTOR itself, and the other one is through the reduction of leucine uptake in the cell, and if you reduce intracellular leucine, mTOR is not as active," Ardehali said.
According to the investigators, the findings suggest that reducing anabolism by reducing iron could be a potential treatment strategy for cancer, which relies on anabolism to grow and spread.
"We think that if you reduce iron in the cancer cells, that can have synergistic effects in inhibiting cancer growth in addition to other chemotherapy agents. That's something we would like to eventually study, by looking at iron chelators as adjunct therapy to other chemotherapies and see if that would have additional benefit in treating cancer," Ardehali said.
"I think we are just scratching the surface when it comes to learning how cells coordinate fundamental life processes with the levels of elemental nutrients, like iron and other metals, and my hope is that our work becomes a springboard for future research in this area," Shapiro said.
More information: Jason S. Shapiro et al, Iron drives anabolic metabolism through active histone demethylation and mTORC1, Nature Cell Biology (2023). DOI: 10.1038/s41556-023-01225-6
Journal information: Nature Cell Biology
Provided by Northwestern University