This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Small-molecule autocatalysis may have paved the way for the emergence of evolution by natural selection
The discipline of systems chemistry deals with the analysis and synthesis of various autocatalytic systems and is therefore closely related to the study of the origin of life, since it investigates systems that can be considered as a transition between chemical and biological evolution: more complex than simple molecules, but simpler than living cells.
Tibor Gánti described the theory of self-replicating microspheres as early as 1978. These still lacked genetic material, but concealed within their membranes an autocatalytic metabolic network of small molecules, isolated (compartmentalized) within their membranes.
As the autocatalytic process takes place, the membrane-building material is also produced, leading to the division of the sphere. This system may appear to be a living cell, and although it lacks genetic material, this can only be verified experimentally. These microspheres can be considered as "infrabiological" chemical systems, since they do not reach the level of biological organization, but they exceed the complexity of normal chemical reactions.
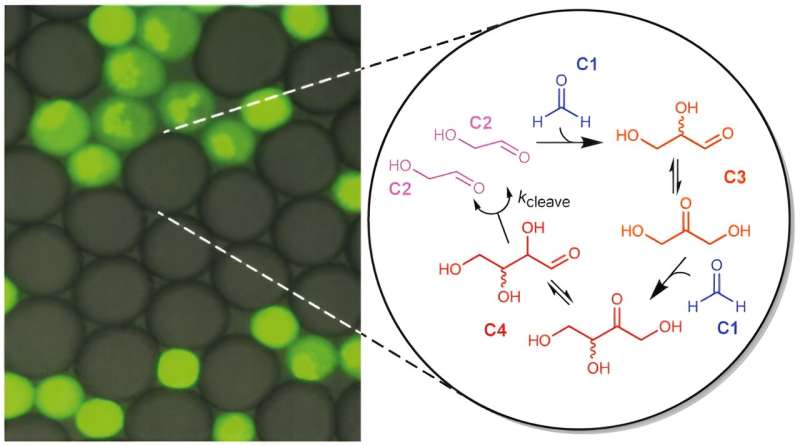
Years ago, we started to think about the possibility of experimentally realizing the process whereby the growth of a small molecule metabolic network leads to the growth of the compartments that enclose the network, to the effect that they can divide. Tibor Gánti has already identified one of the most promising candidates for this system as the formose reaction, an autocatalytic sugar-producing reaction that consumes formaldehyde and involves the circular transformation and propagation of glycolaldehyde molecules. The reaction does not require enzymes.
The study was carried out in the biochemistry laboratory of the École Supérieure de Physique et de Chimie Industrielles (ESPCI) in Paris by Professor Andrew Griffiths and his colleagues. The experiment involved creating tiny water droplets in an oil medium that did not fuse and therefore acted as artificial cells. The work is published in the journal Nature Chemistry.
Some of the "cells" were given glycolaldehyde as an autocatalyst (in addition to formaldehyde as a nutrient), others were not. In the former group, the formose reaction was triggered and, by osmosis, it sucked water away from compartments that did not contain glycolaldehyde. This allowed them to grow and to divide under external influence. Many researchers have suggested that before the emergence of regulated cell division, the initial cells divided in response to external influences such as turbulent flow.
The significance of this study is that we are the first in the world to show that the operation of a network of small-molecule autocatalytic reactions, without genetic material and enzymes, leads to the growth and division of compartments, i.e., the formation of new generations.
This has never been demonstrated before, so the result is fundamental to the experimental verification of the principles of systems chemistry and points the way forward in the study of the origin of life.
More information: Heng Lu et al, Small-molecule autocatalysis drives compartment growth, competition and reproduction, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01276-0
Journal information: Nature Chemistry
Provided by Ökológiai Kutatóközpont