This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Watching a bimetallic catalytic surface in action
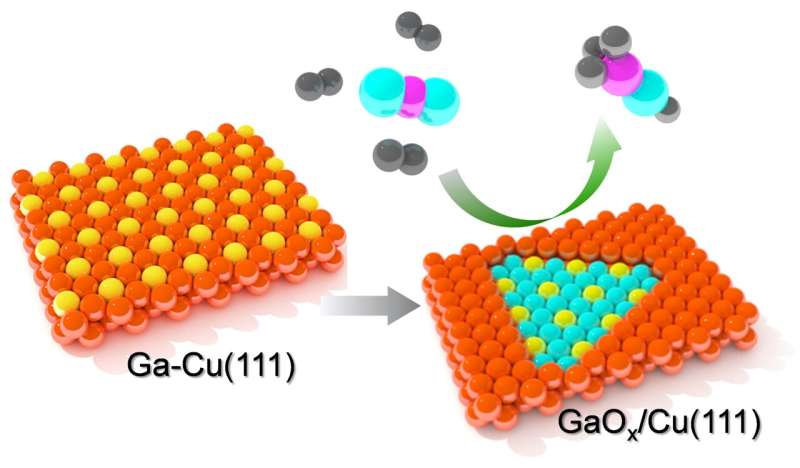
A team of researchers from the Department of Interface Science at the Fritz Haber Institute of the Max Planck Society addressed the question: what happens to a Ga-promoted Cu surface under reaction conditions required for the synthesis of methanol? They found complex structural transformations of this bimetallic catalyst that might change the common view on the catalytically active surface structure.
Hydrogenation of CO2 to methanol occurs with high efficiency on famous Cu/ZnO/Al2O3 catalysts at high pressures, i.e. 50–100 bar. However, this synthesis does not only bring along safety risks and a high energy consumption, but also limits the CO2 concentration in the gas feed in order to maintain high selectivity.
Therefore, a new class of catalysts for low pressure methanol synthesis is highly desirable, also for future development of small-scale devices using solar-generated hydrogen at ambient pressure.
It has recently been discovered that intermetallic compounds and alloys containing Ga show good catalytic performance even at atmospheric pressures. However, the promotional role of Ga in these catalysts is still poorly understood, primarily because of the lack of information about the surface structures of the catalysts.
In this respect, studies using surface-sensitive techniques applied to well-defined model catalysts under reaction conditions can provide key information that will aid our understanding of the dynamic nature of the active sites, reaction intermediates, and ultimately the reaction mechanism.
A team of researchers from the Department of Interface Science at the Fritz Haber Institute took advantage of laboratory-based Near Ambient Pressure X-ray Photoelectron Spectroscopy (NAP-XPS) and Scanning Tunneling Microscopy (NAP-STM), to monitor in situ the structural and chemical evolution of Ga-Cu bimetallic surfaces in the CO2 hydrogenation reaction.
They observed temperature- and pressure-dependent de-alloying of the bimetallic surface resulting in Ga-oxide islands embedded into the Cu surface. Although the oxide phase showed a stoichiometry close to Ga2O3, i.e., the most stable Ga-oxide, it actually forms an ultrathin layer.
The promotional effect of metals like Ga, which are prone to oxidation, is often discussed within structure models where a bulk oxide is placed on top of the metal surface and the corresponding reaction mechanism involves spillover of intermediate species at the interface. The present study clearly demonstrated that: (i) Ga-oxide is embedded into the metal surface; and (ii) the Ga-oxide islands are ultrathin, most likely of "monolayer" thickness.
The reaction -induced formation of ultrathin Ga-oxide layer on metal surfaces is also anticipated for Ga-containing intermetallic compounds. Importantly, such two-dimensional oxide films are very different from their bulk counterparts in terms of structure and reactivity.
Therefore, the GaOx/Cu interface formed under CO2 hydrogenation reaction conditions may expose catalytically active sites never considered for this reaction before. Such information would be impossible to obtain using bulk-sensitive techniques commonly employed for the characterization of powder catalysts.
The results of this study, which were recently published in Nature Communications, shed light on the complex surface structure of Ga-containing catalytic systems.
Such insight on working catalysts is only possible to obtain using state-of-the-art experimental techniques under reaction conditions. Only by establishing the atomic structure of the Ga-oxide layer(s) and its interface to the transition metal under working conditions can one bring insight into the reaction mechanism of this methanol synthesis catalyst.
More information: Si Woo Lee et al, Unraveling surface structures of gallium promoted transition metal catalysts in CO2 hydrogenation, Nature Communications (2023). DOI: 10.1038/s41467-023-40361-3
Journal information: Nature Communications
Provided by Max Planck Society