This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Machine learning enables discovery of DNA-stabilized silver nanoclusters

DNA can do more than pass genetic code from one generation to the next. For nearly 20 years, scientists have known of the molecule's ability to stabilize nanometer-sized clusters of silver atoms. Some of these structures glow visibly in red and green, making them useful in a variety of chemical and biosensing applications.
Stacy Copp, UCI assistant professor of materials science and engineering, wanted to see if the capabilities of these tiny fluorescent markers could be stretched even further—into the near-infrared range of the electromagnetic spectrum—to give bioscience researchers the power to see through living cells and even centimeters of biological tissue, opening doors to enhanced methods of disease detection and treatment.
"There is untapped potential to extend fluorescence by DNA-stabilized silver nanoclusters into the near-infrared region," she says. "The reason that's so interesting is because our biological tissues and fluids are much more transparent to near-infrared light than to visible light."
Copp says scientists and engineers have been looking for new ways of scanning bodily tissues to avoid the mutating side effects of X-rays or having patients ingest radionuclides to detect tumors. "There are lots of reasons why it would be exciting to use noninvasive, nonhazardous near-infrared light, which is basically heat," she says. "But one of the biggest challenges is that we don't really have good, nontoxic fluorophores—molecules or nanoparticles that emit this near-infrared light."
People have been aware of the antimicrobial powers of silver since ancient times. The element kills bacteria but is benign to most mammalian cells; it's even used to fight odors in some fabrics that humans wear. Copp says recent studies have shown that DNA-stabilized silver nanoclusters have low cytotoxicity, and DNA is inherently biocompatible—which makes these compounds potentially safe to use in a clinical setting.
As with many things DNA-related, there is an almost incomprehensible quantity of sequence permutations, only a small subset of which possess the fluorescent qualities researchers seek. While at UC Santa Barbara, Copp was part of a team that designed an instrument that can rapidly scan hundreds of silver nanoclusters at a time to see if they have near-infrared emission. With this tool, the scientists have been able to find a large number of previously hidden candidate sequences.
In her lab in UCI's Susan and Henry Samueli Interdisciplinary Science and Engineering Building, Copp initiated a project with Peter Mastracco, her first Ph.D. student, to take advantage of new data connecting DNA sequences to the colors of the nanoclusters, which Copp says she likens to a "nanocluster genome." She asked Mastracco to develop a machine learning method that could help them analyze mountains of experimental data to come up with novel DNA sequences—ones capable of being created in the lab—that open access to the near-infrared region.
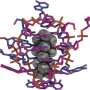
Early in the project, Mastracco found a research paper showing the X-ray crystal structure of a DNA-stabilized silver nanocluster. "It literally gave us a picture of where all the silver atoms are and how the DNA is folded around the nanocluster," Copp says. "And he spotted something I hadn't noticed before, which is that the DNA folded around the nanocluster in a particular way."
The researchers hypothesized that if they encoded information about this folding peculiarity into their machine learning models, they might be able to predict the fluorescence color of the nanoclusters.
Part of Mastracco's Ph.D. training in Copp's group was to become a mentor. In the summer of 2020—an early peak in the COVID-19 pandemic—he was matched with Josh Evans, a student at Chaffey College, a community college with campuses in California's Inland Empire.
According to Copp, Evans devised a creative way to interpret the results of Mastracco's models more clearly. "Some of these algorithms can operate like a black box," Copp says. "You provide a data set to the machine learning algorithm, and it learns the trends in that data, and that helps you make predictions. But it really can be hard to open the lid to find out what's going on in the box."
Evans helped solve this problem by using a "feature selection tool" that allowed the team to determine what part of the DNA sequence was correlated to the different fluorescence colors of the nanoclusters.
Copp says the breakthrough became an essential contribution to a research paper—with Mastracco as lead author—that was published in the journal ACS Nano.
Work in the Copp research group on fluorescent nanoclusters continues apace. They recently published a second paper on the subject in Journal of the American Chemical Society, this one led by Ph.D. student Anna Gonzalez Rosell, who mentored UCI undergraduate co-author Nery Arevalos.
"The paper represents a key advance in developing truly biocompatible nanoclusters for near-infrared imaging," Copp says. "Several of my students worked on these papers, and undergrad mentorship played a vital role in the projects. It's an arrangement that works incredibly well in terms of delivering research results and helping young scientists achieve their goals."
More information: Peter Mastracco et al, Chemistry-Informed Machine Learning Enables Discovery of DNA-Stabilized Silver Nanoclusters with Near-Infrared Fluorescence, ACS Nano (2022). DOI: 10.1021/acsnano.2c05390
Anna Gonzàlez-Rosell et al, Chloride Ligands on DNA-Stabilized Silver Nanoclusters, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c01366
Journal information: Journal of the American Chemical Society , ACS Nano
Provided by University of California, Irvine