This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Protective molecules facilitate molybdate anion binding to create novel silver nanoclusters
Silver nanocluster synthesis, or the binding of a silver atom to negatively charged anions, is often plagued by stability, sizing uniformity and surface composition issues. Protective molecules that bind the surface of silver nanoclusters, called ligands, can mitigate these issues, creating new nanoclusters with unique physical and chemical properties.
Nanoclusters are stable, crystalline materials up to two nanometers in diameter that are engineered for specific properties, such as the acceleration of a particular chemical reaction, as a semiconducting material or for targeted drug delivery.
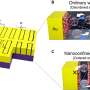
A group of nanoparticle scientists from Mian-Ming Zhang's group at Shanxi Normal University and the Taiyuan University of Technology recently synthesized and characterized a new silver-molybdate nanocluster with the help of thiolate and phosphine ligands to protect the surface of the cluster and increase stability and uniformity. The use of protective ligands will assist other researchers in synthesizing additional silver metal and molybdate anion nanoclusters with unique characteristics in the future.
The team published the results of their study in the May 11 issue of the journal Polyoxometalates.
"In order to further stabilize the cluster during synthesis, or to obtain a diverse cluster structure, a second ligand or a larger variety of ligands is often introduced during nanocluster synthesis. Strategies for mixed ligand protection were chosen in our study. For thiol/phosphine mixed ligands, adding thiol can overcome the single coordination of phosphine to silver as an auxiliary ligand and increase the abundance of coordination with silver," said Jin-Ping Gao, first author of the study paper and researcher at the Key Laboratory of Magnetic Molecules & Magnetic Information Materials at Shanxi Normal University in Taiyuan, China.
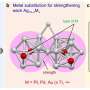
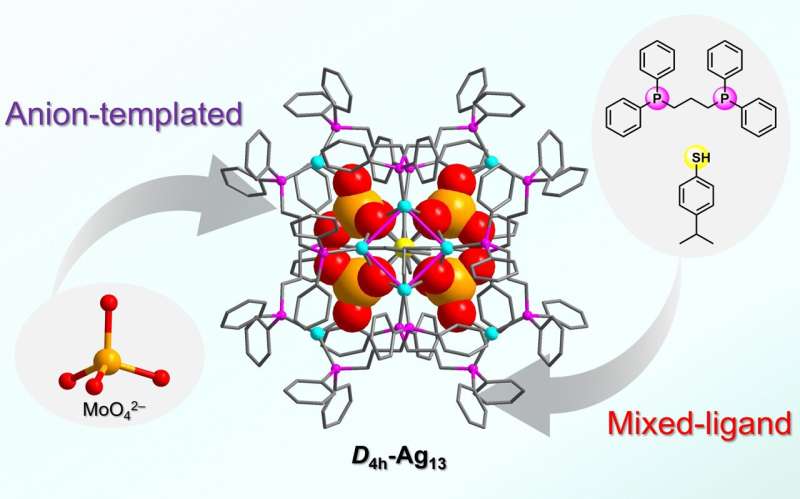
"Here, we introduced raw sodium molybdate… into the reaction process… to guide the synthesis of this quadruple-symmetric 13-silver atom cluster," said Gao. Molybdates are often introduced into the reaction environment and through self-assembly reactions can be obtained as negatively charged molybdenum POMs, which can be encapslated in a silver cluster structure to change their size, shape and balance charge.
"We isolated an ideal, quadruple-symmetric sandwich cationic (positively charged)… nanocluster, [Ag13(MoO4)4(SC6H4iPr)2(dppp)8]3+ (Ag13), which is synthesized using four molybdate [MoO4]2− anions and protected by two ligands, thiolate (iPrC6H4S−) and phosphine (dppp). This work demonstrates that simple molybdate anions also have the potential to induce novel structures, opening the door to the use of this anion to isolate structures of interesting clusters," said Gao.
Nanocluster synthesis is paramount to developing new cluster structures with different chemical and physical properties. "Metal nanoclusters are ideal nanomaterials for applications in different fields, including catalysis, or speeding chemical reactions, and biological applications such as bioimaging, sensing, optics, imaging, biolabeling and targeted drug delivery," said Gao.
The research team looks forward to overcoming new silver nanocluster synthesis challenges to create the nanomaterials of the future. This work will include experimenting with various protective ligands and understanding how peripheral organic molecules and inorganic cores are bonded to determine their precise chemical structure.
"We aspire to generate more silver clusters with high-symmetry aesthetics using a strategy of mixed-ligand combinations induced by an anionic, or negatively charged, template in the future. Our goal is to enrich the research process of mixing strategies to construct novel silver clusters and broaden the research progress of molybdate in highly symmetric silver clusters," said Gao.
More information: Jin-Ping Gao et al, [MoO 4] 2−-templated D 4 h -symmetric sandwich Ag 13 nanocluster coprotected with thiolate and phosphine, Polyoxometalates (2023). DOI: 10.26599/POM.2023.9140028
Provided by Tsinghua University Press