This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
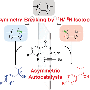
Aggregation-induced catalysis: Asymmetric catalysis with chiral aggregates
Asymmetric synthesis and catalysis have been actively pursued in chemical and materials sciences for some time. Increasing numbers of drugs and pesticides contain chiral structural units in their structures since drug actions require conformational matching to increase their potency and selectivity toward receptors and other active targets inside and on the surfaces of cells.
Structural design of pharmaceuticals plays a key role in reducing or avoiding severe side effects during their action processes. In the meanwhile, more advanced materials, especially nano and photoelectronic materials contain micro-and macro-chiral units requiring various levels of asymmetric factors. Therefore, the control of chirality will continue to play a crucial role in the aforementioned fields.
There have been four methods to control chirality including the use of chiral auxiliaries, reagents, solvents, and catalysts documented in literature and textbooks. Among them, asymmetric catalysts are normally divided into homogeneous and heterogeneous catalysis.
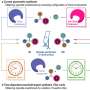
The Texas Tech University/Nanjing University Li Guigen team reports a new method for controlling chirality—asymmetric synthesis (AIAS) and asymmetric catalysis (AIAC) using chiral aggregates. This method is different from the five reported asymmetric synthesis methods: chiral auxiliary, chiral reagents, chiral solvents and chiral catalysts. These five methods are basically based on the reaction behavior of individual molecules in the reaction solution.
The aggregation-induced asymmetric catalysis (AIAC) method is different from traditional homogeneous catalysis (single molecular behavior) and heterogeneous catalysis. It is a new type of asymmetric catalysis in between. AIAS is through the aggregation of chiral auxiliary raw materials, AIAC is through the aggregation of chiral catalysts to achieve the purpose of controlling chirality.
Both of the new methods can be used to react with low or non-toxic solvents, and can achieve the purpose of improving stereoselectivity or controlling the opposite chiral configuration.
This work not only presented a new concept, but also confirmed it in asymmetric reaction processes through direct observation and evidence of molecular aggregation.
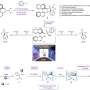
The Sharpless asymmetric catalytic dihydroxylation reaction (AD) was selected to represent this new concept and method owing to its simplicity with predictable asymmetric control by well-known and commercially available chiral ligands. The commercial AD-mixes containing the ligands (DHQ)2PHAL and (DHQD)2PHAL, referred to as AD-mix-α and AD-mix-b, respectively, were directly used for the reaction at room temperature.
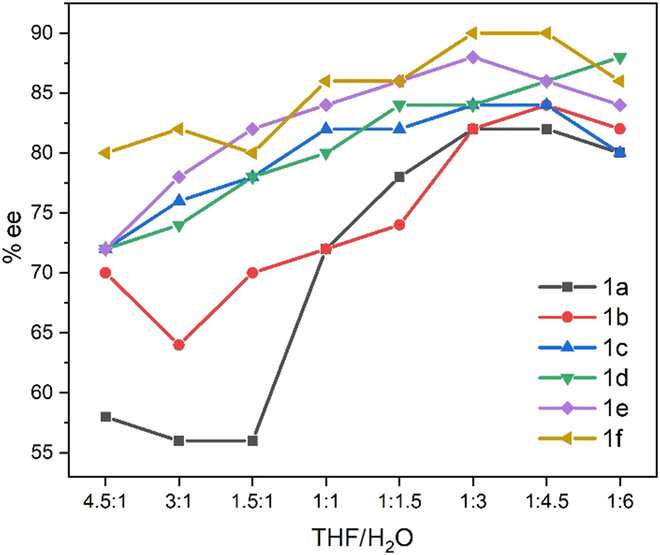
For the sake of aggregation, the co-solvents of THF-H2O commonly adopted in AIE (aggregation-induced emission) and AIP (aggregation-induced polarization) were employed for the present study instead of the traditional AD solvents of acetone, acetonitrile, alcohols together with water. Among many AD substrates, styrene and its derivatives were considered since they are best researched mechanistically in AD due to their simplicity.
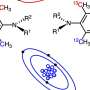
The correlation of chiral aggregates to aggregation-induced polarization (AIP) was further explored to render optical rotation enhancement or adjustment as more polar solvent was added in co-solvents. AIP measurements of chiral dimeric quinine ligands were conducted using a Rudolph polarimeter (Rudoph Research Analytica APIV/2W) with the sodium lamp emitting light at 589 nm as the light source.
The samples were prepared at a consistent concentration (c = 2 mg/mL) in the cosolvents commonly employed AIE, and also for the present asymmetric dihydroxylation were thus utilized. The average specific rotation values taken from three measurements were considered the most authentic results and further plotted to relation curves with specific rotation on the Y axis.
The water fraction was set at the component of 5% (v/v) on the X horizontal axis. (DHQD)2PHAL demonstrated a substantial increase trend in the specific optical rotation values with gradually increased water fractions, from -186.5o to -132.5o during the water fraction's changing from 0% to 65%. In contrast, (DHQ)2PHAL showed an opposite trend, which featured a consistent descending trend, in which the optical rotation value dramatically fell from 292.5o to 250 o.
This strategy demonstrated promising results on other reactions. For example, in the same co-solvent system containing THF and H2O, organocatalytic asymmetric Diels-Alder reaction between 1,3-diphenylisobenzofuran and (E)-but-2-enal depicted opposite enantioselectivity with respect to endo-isomer by increasing fw values. This aggregation strategy would serve as a greener and more environmentally friendly tool of controlling chirality of products as compared with classical methods aforementioned.
In the latter, in order to generate products of opposite chirality, the chirality of reactants, as well as catalysts or chiral solvents, needs to be reversed by performing synthesis of these materials, causing manpower and energy and producing more waste generation.
The research is published in the journal Research.
More information: Yao Tang et al, Aggregation-Induced Catalysis: Asymmetric Catalysis with Chiral Aggregates, Research (2023). DOI: 10.34133/research.0163
Provided by Research