This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Synthesis of peripherally annulated phenanthroporphyrins
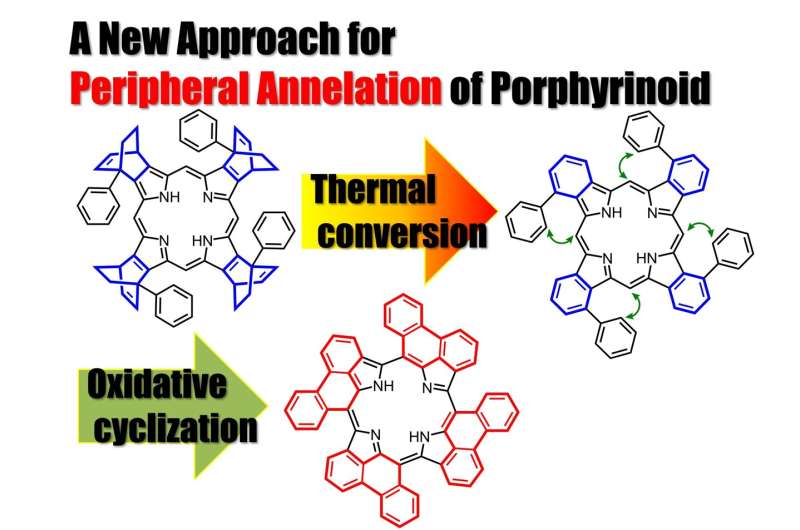
Prof. Okujima, in collaboration with Prof. Kobayashi at Shinshu University, have reported the synthesis, molecular structure, optical properties and electronic structure of unusual phenanthrene-fused porphyrins in Organic Letters.
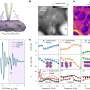
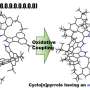
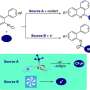
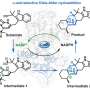
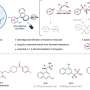
Precursor porphyrins fused with aryl-substituted bicyclo[2.2.2]octadiene afforded the corresponding arylbenzoporphyrins (arylBPs) by retro Diels–Alder reaction. Unusual phenanthroporphyrins were obtained via the intramolecular Scholl reaction of arylBPs.
The researchers analyzed the optical and electronic structures using magnetic circular dichroism spectroscopy and time-dependent density functional theory calculations.
More information: Kota Muramatsu et al, Synthesis of Peripherally Annulated Phenanthroporphyrins, Organic Letters (2023). DOI: 10.1021/acs.orglett.3c00876
Journal information: Organic Letters
Provided by Ehime University