This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Two-dimensional nanoparticles with great catalytic potential
Hydrogen is considered an environmentally friendly alternative to conventional fossil fuels. Until now, expensive and rare substances such as platinum have been needed for its catalytic production, for example via electrolytic water splitting. More readily available catalysts could make the production of large quantities possible in the future.
The research teams of Helmut Cölfen (Physical Chemistry) and Peter Nielaba (Statistical and Computational Physics) at the University of Konstanz have developed a general method to produce two-dimensional nanoparticles from readily accessible materials, together with researchers from the Ocean University of China, Qingdao (China) and the Fritz Haber Institute of the Max Planck Society, Berlin (Germany).
Two-dimensional nanoparticles have a high catalytic potential, which is why this synthetic route is suitable for producing particularly active catalysts.
The corresponding synthesis process is carried out in a simple aqueous solution. No toxic additives or particularly high temperatures, which are energetically unfavorable, are required. The process is controlled by simply varying the concentration of the components and by temperature regulation. The research team succeeded in shaping more than 30 different compounds into two-dimensional forms using this method, which has been described now for the first time in the journal Nature Synthesis.
The advantage of two-dimensional nanoparticles
Two-dimensional (2D) nanoparticles have a particularly large number of surface atoms, which have different properties than atoms within a particle. The bonds of the surface atoms are non-saturated because the surface lacks the immediate neighboring atoms to which bonds are formed inside the particle. This leads to surface or interfacial tension. Since this non-saturated state is quite energy consuming for the overall system, nanoparticles try to cluster together to saturate the bonds and minimize the surface area.
However, if the surface bonds remain unsaturated, this results in increased chemical reactivity. The number of unsaturated bonds is particularly high in two-dimensional nanoparticles because they have unsaturated bonds not only at the top and bottom, but also at the sides and edges. This makes them particularly interesting for catalysis, which plays a major role in chemistry. However, the required nanocrystals are difficult to fabricate because of the unfavorable energy state at the surface.
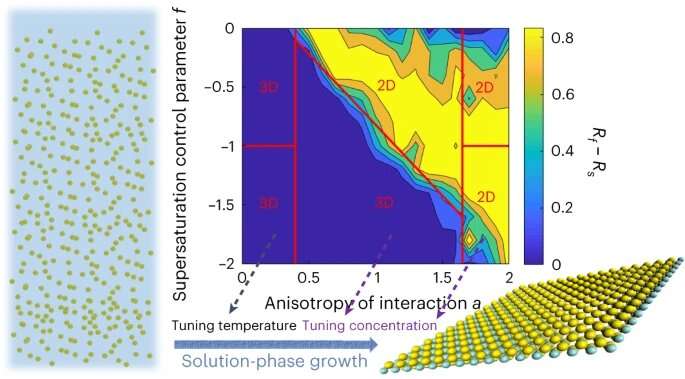
Two-dimensional nanoparticles are anisotropic, and their properties depend on the orientation of their building blocks. The crystal lattice of the particles is decisive for their growth direction. If the nanoparticles have a layered crystal lattice as in clay, the particles grow two-dimensionally. However, materials that are favorable for catalysis rarely adopt the two-dimensional shape on their own.
If the crystal lattice dictates that the crystal grows rapidly along two crystal axes, two-dimensional nanoparticles can be easily synthesized. Then, only a few molecular building blocks are needed in the solution to grow the nanoparticles two-dimensionally. If the crystals grow in other directions just as fast or only slightly slower, the crystals take on a three-dimensional shape.
How nanoparticles grow two-dimensionally
The research team has discovered how the concentration of molecular building blocks in the solution can be used to manipulate this process: If the concentration of building blocks is increased, the principle of "what grows fast also consumes more material" comes into play: The distance between the fast-growing and the slower growing crystal axes increases, resulting in two-dimensional particles.
The method of increasing the building block concentration does not work if the growth rate along different relevant crystal axes is approximately the same. In this case, the researchers use another parameter. The growth rate of crystal surfaces depends exponentially on temperature. If the temperature of the solution is changed by even a few degrees, the difference in the growth rate between the slow and fast-growing crystal faces will increase. As a result, the nanoparticles grow in two dimensions.
Method works for over 30 elements of the periodic table
This general procedure works for many materials. In the periodic table, the German-Chinese research team was able to identify metals in many groups, more than 30 in total, which take the two-dimensional form as oxides or hydroxides, but also acids, sulfides, oxychlorides and phosphates. The advantage of this general approach, which has been described for the first time: In most cases, the materials are produced at room temperature in water—without toxic solvents or high temperatures.
Moreover, the yield of catalytic materials is highly scalable. In the lab, the researchers are working on a multigram scale. To produce catalysts in large quantities using easily accessible substances, all that is needed is a sealed vessel—instead of special apparatuses such as pressure vessels.
Experiments confirm theory
The experimental study also shows how theoretical knowledge can be put into practice. The experiments confirm theoretical simulations performed by Peter Nielaba's team in a joint project with the Cölfen team in the Collaborative Research Centre 1214 "Anisotropic Particles as Building Blocks: Tailoring Shape, Interactions and Structures" at the University of Konstanz.
The physicist had already taken into account variations in the concentration of the components and the temperature. "The calculations and what we have found experimentally completely coincides," concludes Helmut Cölfen.
The work is published in the journal Nature Synthesis.
More information: Zongkun Chen et al, Growth strategy for solution-phase growth of two-dimensional nanomaterials via a unified model, Nature Synthesis (2023). DOI: 10.1038/s44160-023-00281-y
Journal information: Nature Synthesis
Provided by University of Konstanz