This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Low concentration CO2 can be reused in biodegradable plastic precursor using artificial photosynthesis
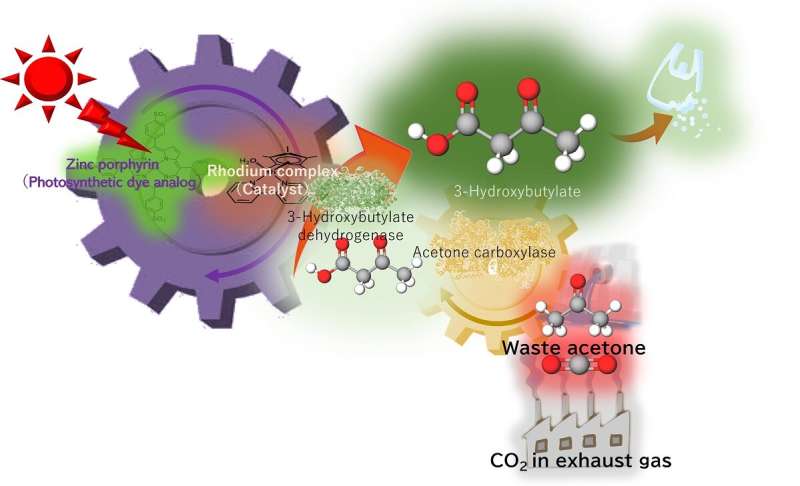
Osaka Metropolitan University scientists have developed a process using artificial photosynthesis to successfully convert more than 60% of waste acetone into 3-hydroxybutyrate, a material used to manufacture biodegradable plastic. The results were obtained using low-concentration CO2, equivalent to exhaust gas, and powered by light equivalent to sunlight for 24 hours.
The researchers expect that this innovative way of producing biodegradable plastic could not only reduce CO2 emissions but also provide a way of reusing laboratory and industrial waste acetone. Their findings have been published in the journal Green Chemistry.
Poly-3-hydroxybutyrate—a biodegradable plastic—is a strong water-resistant polyester often used in packaging materials, made from 3-hydroxybutyrate as a precursor. In previous studies, a research team led by Professor Yutaka Amao from the Research Center for Artificial Photosynthesis at Osaka Metropolitan University found that 3-hydroxybutyrate can be synthesized from CO2 and acetone with high efficiency, but this was only demonstrated at higher concentrations of CO2 or sodium bicarbonate.
This new study aimed to reuse waste acetone from permanent marker ink and low concentrations of CO2—equivalent to exhaust gas from power plants, chemical plants, or steel factories. Acetone is a relatively inexpensive and reasonably harmless chemical used in many different laboratory settings, either for reactions or as a cleaning agent, which produces waste acetone. The acetone and CO2 acted as raw materials to synthesize 3-hydroxybutyrate using artificial photosynthesis, powered by light equivalent to sunlight.
"We focused our attention on the importance of using CO2 created by exhaust gas from thermal power plants and other sources to demonstrate the practical application of artificial photosynthesis," explained Professor Amao.
After 24 hours, more than 60% of acetone had been successfully converted to 3-hydroxybutyrate. "In the future, we aim to develop artificial photosynthesis technology further, so that it can use acetone from liquid waste and as well as exhaust gas from the laboratory as raw materials," stated Professor Amao.
More information: Yu Kita et al, Visible-light-driven 3-hydroxybutyrate production from acetone and low concentrations of CO2 with a system of hybridized photocatalytic NADH regeneration and multi-biocatalysts, Green Chemistry (2023). DOI: 10.1039/D3GC00247K
Journal information: Green Chemistry
Provided by Osaka Metropolitan University