This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop novel alkaline anion exchange membrane
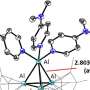
![The complex of [2.2.2]cryptand and Ba2+ is used as a novel cationic functional group for alkaline anion-exchange membrane applications, which synergistically has high binding constant, excellent alkaline stability, and a stable main-group-metal center. Credit: Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202217742 Researchers develop novel alkaline anion exchange membrane](https://scx1.b-cdn.net/csz/news/800a/2023/researchers-develop-no-2.jpg)
As the central component of alkaline exchange membrane fuel cells (AEMFCs) and alkaline exchange membrane water electrolyzers (AEMWEs), the development of alkaline anion exchange membranes (AAEMs) is critical to the global sustainable hydrogen energy landscape. AAEMs are polyelectrolytes transferring hydroxide anions (OH-), consisting of a polymer backbone and cationic functional groups.
However, the strong nucleophilicity and basicity of OH- anions result in alkaline instability for AAEMs. For example, organic cations and transition-metal-ligand-complexes, which are currently widely used, are prone to degradation by nucleophilic substitution, Hoffman elimination, and cationic redox during the service period. The above mentioned alkaline instability has become the major factor limiting the product lifetime. To date, it is still challenging to incorporate suitable cationic functional groups into the molecular chain to further improve the alkaline stability of AAEMs.
In a recent study published in Angewandte Chemie International Edition, Prof. You Wei and Assoc. Prof. Wang Yu from the Institute of Chemistry of the Chinese Academy of Sciences (ICCAS) and Prof. Zhang Wenjuan from Beijing Institute of Fashion Technology (BIFT) designed and developed olefin polymers with [2.2.2]cryptand of complexed metal cations as functional cationic groups for AAEM applications.
[2.2.2]Cryptand possess good chemical stability and strong binding with Ba2+ (Ks over 109 M-1 in water at 25 °C). Cryptand groups can be grafted onto polyolefin backbones by post-polymer modification or introduced directly by ring-opening metathesis polymerization. The barium [2.2.2]cryptate complex ([Cryp-Ba]2+) AAEMs with polyolefin backbones remain stable after treatment in 15 M KOH at 60 °C for more than 1,500 hours, while the conductivity of commercial membrane PiperION A80 rapidly decreased by over 90% after 360 h.
This cryptand modified polyolefin ion exchange membrane was also successfully applied to AEMWEs, and the effect of cation binding strength was further clarified by monitoring the resulting performance changes based on the switching anode feeding solution.
This work confirms that the binding constant between the metal and the anchoring group is an important criterion to determine whether the polyelectrolyte membranes are real AAEMs, and provides a new idea to solve the alkaline stability problem of AAEMs.
More information: Haixia Zhang et al, Alkaline‐Stable Anion‐Exchange Membranes with Barium [2.2.2]Cryptate Cations: The Importance of High Binding Constants, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202217742
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences