This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers find access to new fluorescent materials
Fluorescence is a fascinating natural phenomenon. It is based on the fact that certain materials can absorb light of a certain wavelength and then emit light of a different wavelength. Fluorescent materials play an important role in our everyday lives, for example in modern screens. Due to the high demand for applications, science is constantly striving to produce new and easily accessible molecules with high fluorescence efficiency. Chemist Professor Evamarie Hey-Hawkins from Leipzig University and her colleagues have specialized in a particular class of fluorescent materials—phospholes.
These consist of hydrocarbon frameworks with a central phosphorus atom. In experiments with this substance, Nils König from Hey-Hawkins' working group has found access to new fluorescent materials. He has now published his findings in the journal Chemical Science.
"Phospholes can be modified by certain chemical reactions, which has a major impact on the color and efficiency of the fluorescence of the molecule. Another special feature of these substances is their propeller-like structure," explains König. When these molecules are dissolved in a solvent and exposed to UV light, they do not fluoresce. The absorbed energy is released in the form of rotational motion, causing the molecules to spin like a propeller in the solvent. In a crystalline state, however, the ability to rotate is severely limited, which makes the substances fluoresce strongly under UV light. This behavior is known as aggregation-induced emission (AIE).
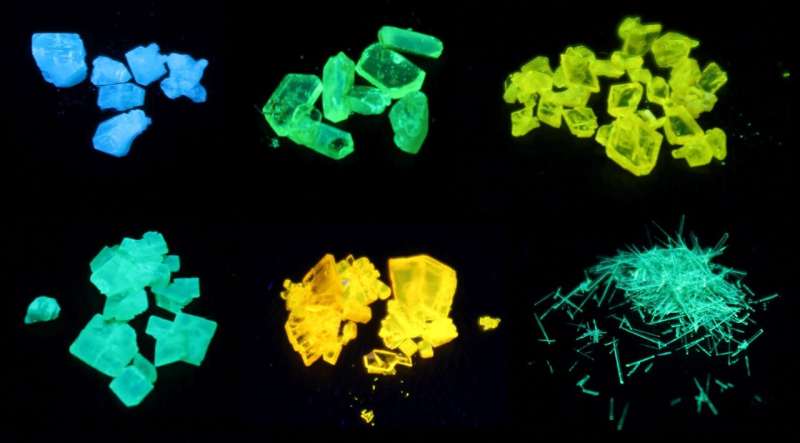
In the recently published paper, Nils König and his colleagues demonstrated a new reaction on AIE-based phospholes, which provided access to a new class of substances. Phospholes can be modified under mild conditions by isocyanates, a reactive class of substances consisting of the elements nitrogen, oxygen and carbon, which are inexpensive and widely available due to their industrial applications in the field of polymers and biochemistry. This reaction, which seems to contradict classical organic chemistry, is characterized by high yields and excellent atom economy.
The optical properties of the new substances were investigated in collaboration with the Institute of Surface Engineering (IOM) in Leipzig, as well as the Center for Nanotechnology (CeNTech) and the University of Münster (WWU). It turned out that the simple modification significantly increased the efficiency of fluorescence compared to the original substances. This is due to the formation of a unique interaction between parts of the molecular framework, which significantly strengthens the molecule in the solid state and leads to stronger fluorescence. The new modification method thus makes a major contribution to understanding the AIE concept and could serve as a tool for synthesizing efficient new dyes for screens or as markers for biomolecules.
More information: Nils König et al, Facile modification of phosphole-based aggregation-induced emission luminogens with sulfonyl isocyanates, Chemical Science (2023). DOI: 10.1039/D3SC00308F
Journal information: Chemical Science
Provided by Leipzig University