February 21, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new encapsulating method for the stabilization of sensitive organolithium reagents
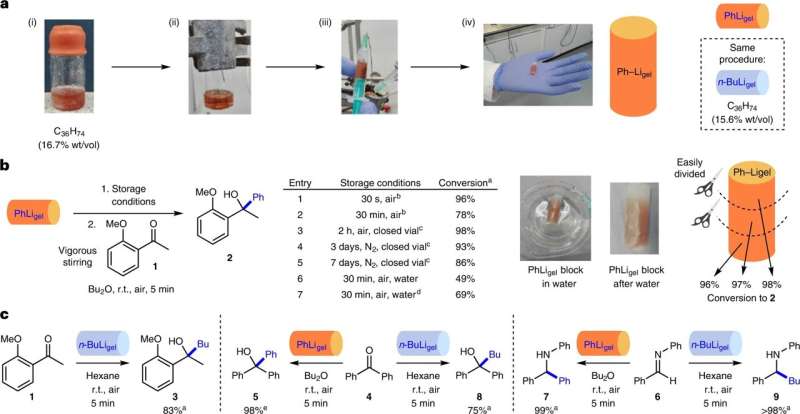
A team of chemists at the University of York has developed a low-cost, encapsulating hexatriacontane organogel that enhances the stability, handling, delivery, storage and portioning of sensitive reagents, making them safer and more accessible for use in synthetic chemistry applications.
In their paper published in the journal Nature Chemistry, the team describes their organogelator, which is not reliant on hydrogen bonds when forming stable structures. Andreu Tortajada and Eva Hevia with the University of Bern have published a News & Views piece in the same journal issue, outlining the work done by the team in the U.K.
Organolithium reagents are chemical compounds with carbon-lithium bonds. They play an important role in organic synthesis, such as in the creation of polymers, agrochemicals and pharmaceuticals. Their highly reactive nature, which makes them so popular as a reagent, has a downside, however; they can only be used under certain conditions such as very low temperatures. They also cannot be exposed to air or moisture when stored—they degrade very quickly.
To overcome the difficulties in handling and using sensitive organolithium reagents, the research team used a 36-carbon-atom-long aliphatic chain, a type of hexatriacontane, to create a gel with properties conducive to protecting sensitive reagents. They tested various concentrations of the gel in which they dissolved samples of the organolithium reagents PhLi and nBuLi during exposure to various environmental conditions.
Initial testing of the gel showed it remained stable after long exposure to air, losing little of its effectiveness after 25 days. It also proved to be tolerant of moist air conditions for up to 30 minutes—long enough to conduct most desired reactions. The research team also found that the gel had both liquid-like and solid-like behavior, allowing for use as an encapsulation gel and also for use during reactions. They found that after such reactions had concluded, the inert components of the gel could be easily removed using standard filtration methods.
The team demonstrated the usefulness and versatility of the gel by encapsulating several sensitive reagents and also using the gel during reactions. They found that not only did the gel perform as expected in stabilizing the reagents during storage, it also did not reduce yields during reactions that were conducted in moist air conditions at room temperatures.
More information: Petr Slavík et al, Organogel delivery vehicles for the stabilization of organolithium reagents, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01136-x
Andreu Tortajada et al, Stable organolithium gels, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01143-y
Journal information: Nature Chemistry
© 2023 Science X Network