This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Origin of endothelial cells constituting the vascular niche for hematopoietic stem and progenitor cells in zebrafish
Endothelial cells (ECs) line blood vessels and can serve as specialized vascular niches for hematopoietic stem and progenitor cells (HSPCs), a special environment where HSPCs reside and self-renew. A team of researchers found that endoderm-derived ECs contribute to zebrafish's functional vascular niche for HSPCs and proposes a new concept that endothelial specialization in the HSPC niche is determined, at least partially, by the origin of the ECs.
The team published their findings in the journal Developmental Cell.
The circulatory system supplies the vertebrate body with oxygen, nutrients, and hormones and removes waste products. ECs constitute the circulatory system by lining the most inner layer of lumenized vessels. They also serve as specialized vascular niches that provide instructive signals to tissue-specific stem cells or parenchymal cells for tissue formation and repair. It is becoming apparent that ECs acquire great specialization and heterogeneity to execute tissue-specific functions.
However, little is known about how organotypic EC specialization and heterogeneity are acquired during development. Growing evidence indicates that they can be determined, at least in part, by local microenvironmental cues, including growth factors, extracellular matrix, and mechanical forces. However, it remains uncertain whether the origin of tissue-specific ECs also plays a role.
Specialized ECs, besides those lining lumenized vessels, constitute an instructive niche for HSPCs. HSPCs are multipotent cells that can self-renew and differentiate to give rise to all blood cell lineages. In hematopoietic tissues, a group of ECs are highly specialized and constitute the vascular HSPC niche to support HSPC maturation, expansion, and differentiation. However, little is known about EC heterogeneity in hematopoietic tissues. In addition, it is still unclear when and how the fate of HSPC niche-constituting ECs is determined.
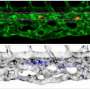
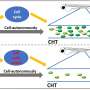
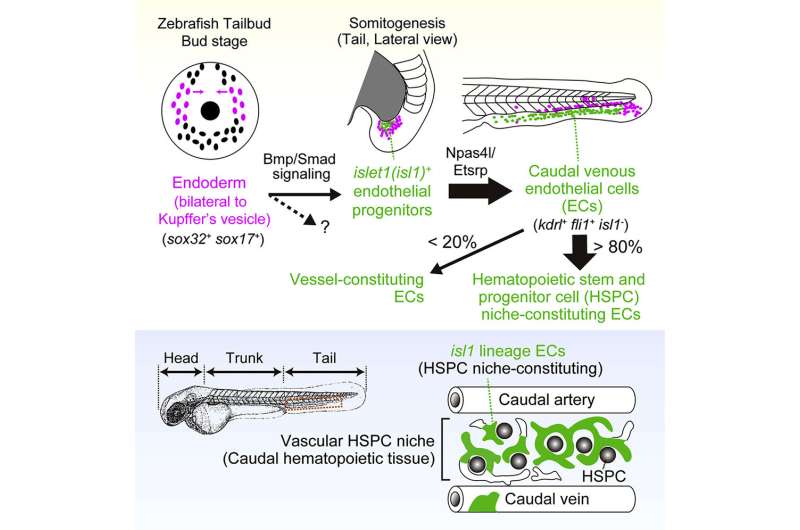
The team identified an unexpected origin for the vascular HSPC niche. They found that islet1-expressing cells are the progenitors of the venous ECs that constitute the majority of the HSPC niche. These islet1-expressing cells surprisingly originate not from the mesoderm, the germ layer classically defined to give rise to ECs, but from the endoderm. The team visualized endothelial differentiation step-by-step from the endoderm to the HSPC niche-constituting ECs via islet1+ endothelial progenitors by live imaging-based lineage tracing.
When these islet1-lineage ECs were specifically ablated, most of the HSPCs were lost from the caudal hematopoietic tissue (CHT), a transient vascular niche for HSPCs in zebrafish. The present results clearly demonstrate that ECs originating from the endoderm via islet1+ endothelial progenitors play a specialized role in forming a functional vascular HSPC niche. Therefore, this study provides evidence that the origin of ECs, at least in part, determines their specialization and heterogeneity in the HSPC niche in zebrafish.
In this study, single-cell RNA-sequencing analyses show that islet1+-derived ECs express a set of genes that reflects their unique origin even as they become heterogeneous. The data strongly suggests that a gene signature of their origin is memorized in the islet1+-derived ECs even after their differentiation to ECs. Therefore, the findings are key to understanding how a lineage-dependent cue regulates EC heterogeneity in future studies.
More information: Hiroyuki Nakajima et al, Endoderm-derived islet1-expressing cells differentiate into endothelial cells to function as the vascular HSPC niche in zebrafish, Developmental Cell (2023). DOI: 10.1016/j.devcel.2022.12.013
Journal information: Developmental Cell
Provided by National Cerebral and Cardiovascular Center