One catalyst generates four nitrogen-containing products at high selectivities

Electrochemists at RIKEN have found a way to selectively convert nitrites into one of four other nitrogen-containing compounds using the same catalyst. This could help to replace fossil-fuel-intensive industrial processes with electrochemical ones powered by renewable energy.
Carbon dominates the news these days, and there is a high public consciousness of the problems ensuing from the disruption of the natural carbon cycle due to the combustion of fossil fuels. But nitrogen, carbon's neighbor on the periodic table, receives far less attention, despite the many environmental problems arising from the disturbance of its natural cycle.
"The nitrogen cycle has been seriously understudied," says Ryuhei Nakamura of the RIKEN Center for Sustainable Resource Science. "But it is critically important for agriculture, and its disruption can cause major ecological problems such as harmful algal blooms, and air and water pollution."
A major cause of these problems is that the industrial processes used to produce fertilizers and other important nitrogen-containing compounds drive the cycle in one direction only, disrupting its balance. "To restore balance, industrial processes are needed that can selectively produce nitrogen-containing compounds in different parts of the cycle," says Nakamura. "But so far this has proved difficult to realize in practice."
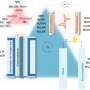
Now, Nakamura and his co-workers have demonstrated an electrocatalytic process using a single catalyst that can convert nitrites into nitrogen monoxide (NO), nitrous oxide (N2O), molecular nitrogen (N2) or ammonium (NH4+) depending on three easily varied experimental parameters.
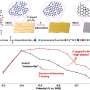
Importantly, these target compounds were produced at high percentages (selectivities) that rival those obtained by specific catalysts optimized for a single target compound.
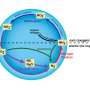
The secret to the team's success was the ability to transfer protons and electrons separately rather than simultaneously. Conventionally, electrons and protons are swapped at the same time in electrocatalytic reactions, but the team adopted a novel strategy that allowed electrons and protons to be exchanged one after the other. This enabled them to tailor the conditions for each transfer.
"This new approach provides much greater control over reactions, and there is a lot of potential to apply it to other chemical systems," says Nakamura. "Our work demonstrates that it's possible to achieve high control over product selectivity even when using a single catalyst for a complex reaction network."
Nakamura notes that the ability to produce fertilizers from renewable energy is particularly important for Japan in light of the country's high dependence on imported fertilizers and low self-sufficiency in food production.
The paper is published in the journal Nature Catalysis.
The team now intends to apply the electrochemical regulation of the nitrogen cycle to fish farms, where the accumulation of nitrates and nitrites on the seafloor causes red tides.
More information: Daoping He et al, Regulation of the electrocatalytic nitrogen cycle based on sequential proton–electron transfer, Nature Catalysis (2022). DOI: 10.1038/s41929-022-00833-z
Journal information: Nature Catalysis
Provided by RIKEN