New research reveals how genes turn on and off
Yeast, that simple organism essential to making beer and bread, has revealed for Cornell University researchers a key mechanism in how genes are controlled.
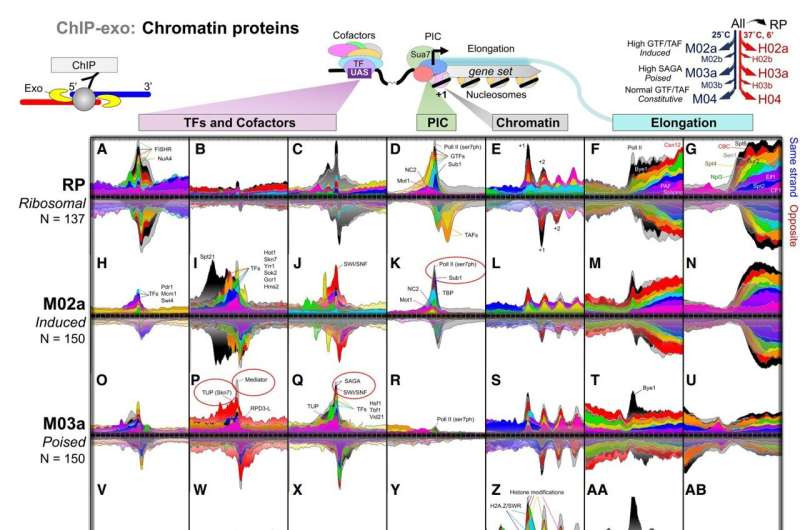
Gene transcription—the elaborate process that our cells use to read genetic information stored in DNA—was long thought to be turned on only when certain regulatory factors traveled to specific DNA sequences. In new research, a team of Cornell scientists discovered that certain genes have their transcription regulatory factors and cofactors already in place, but in a latent state. With the appropriate signals, these "poised" genes become highly active.
Using CRISPR techniques, the researchers removed parts of the yeast transcription machinery to systematically examine the role they play in regulating genes. Yeast and humans have mostly the same molecular machinery to regulate their genes, so yeast provides an excellent model for understanding gene regulation in humans.
"It's like the game of Jenga, where you remove a wood block from a tower of blocks and see if the whole thing crashes down. That's how we learn how protein machines work inside cells," said B. Franklin Pugh, professor of molecular biology and genetics and corresponding author of the study.
"The value of being poised is that certain genes, like environmental response genes, can rapidly respond to a changing environment; for example, when yeast encounters and metabolizes bread sugars, causing the bread dough to rise," Pugh said.
"Building upon years of existing research and combining them with modern and elegant genomics tools helped us in filling gaps in the current knowledge as well as in making new discoveries," said Chitvan Mittal, first author and research associate at the Baker Institute for Animal Health in the College of Veterinary Medicine.
The research was published in Genes & Development.
More information: Chitvan Mittal et al, An integrated SAGA and TFIID PIC assembly pathway selective for poised and induced promoters, Genes & Development (2022). DOI: 10.1101/gad.350026.122
Journal information: Genes & Development
Provided by Cornell University