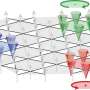
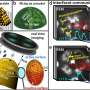
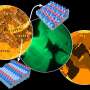
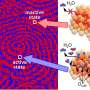
Three microscopes see more than two
One has to look very closely to understand what processes take place on the surfaces of catalysts. Solid catalysts are often finely structured materials made of tiny crystals. There are various microscopies to monitor chemical processes on such surfaces—they use, for example, ultraviolet light, X-rays or electrons. But no single method alone provides a complete picture.
This is why research teams from TU Wien and the Fritz Haber Institute in Berlin have developed a novel approach that allows to have "triple eyes" on a catalytic reaction—using three different surface microscopies. This way, they were able to show that during the catalytic conversion of hydrogen and oxygen to water, reaction fronts on the crystal surface not only form remarkable geometric patterns, but also a new mechanism of the propagation of these fronts was discovered.
For climate-relevant technologies such as ecologically clean hydrogen-based energy production, a comprehensive understanding of such processes is crucial.
Different measurements in a single instrument
"Many scientific questions can only be answered by combining different microscopy methods on the same sample, which is called correlative microscopy," says Prof. Günther Rupprechter from the Institute of Materials Chemistry at TU Wien. "However, this usually comes with limitations."
You have to remove a sample from one instrument and perform the same experiment again in another microscope. Often, for methodological reasons, the experimental conditions are then completely different—some measurements take place in a vacuum, others in air. Often temperatures are different. In addition, you may not be looking at the same spot on the sample with different instruments—this can also influence the results. Thus, it is difficult to combine the results of different measurements in a reliable way.
Ultraviolet, X-rays and electrons
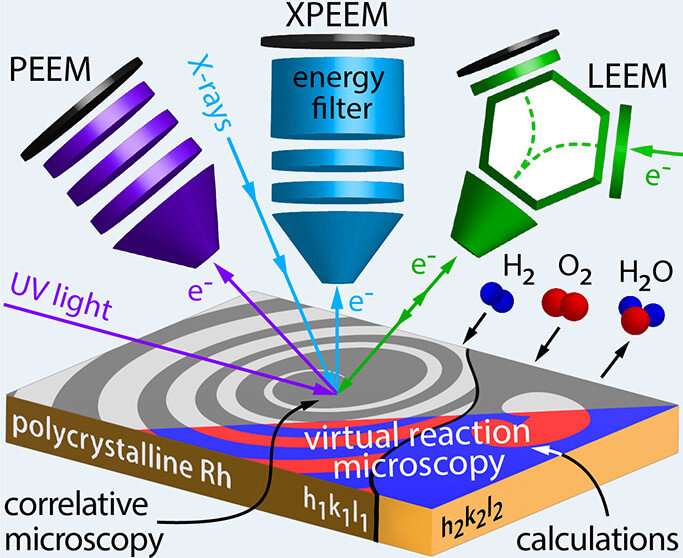
Now, however, it has been possible to combine three different microscopies in a way that the same spot on the same sample was examined under the same environmental conditions. Three different electron microscopies were used: two different variants of photoemission electron microscopy (PEEM), namely UV-PEEM and X-PEEM, and low energy electron microscopy (LEEM).
In UV-PEEM and X-PEEM, the sample surface is illuminated with ultraviolet light and X-rays, respectively. In both cases, it results in electrons being emitted from the surface. Similar to how light beams are focused in an optical microscope, the electron beams form a real-time image of the surface and of the processes taking place there.
In an X-PEEM, one can additionally filter the emitted electrons according to their energies and thereby determine the chemical composition of the sample surface. Access to the necessary high-energy, high-intensity X-rays was provided to the research team by the Berlin synchrotron (HZB BESSY II). In the LEEM technique, the surface is irradiated with an electron beam. The electrons that are backscattered from the surface create the real time image of the sample surface and of the ongoing processes, such as a catalytic reaction.
Since all three microscopies use different imaging mechanisms, this allowed to study different aspects of catalytic hydrogen oxidation on a structurally identical site of the sample , says Prof. Yuri Suchorski, who has been involved in surface microscopy since 1974. "In addition, the X-PEEM technique provides chemical contrast and therefore allows us to correlate the pattern formation on the surface with the chemical composition of the surface and the reactants present on the surface, hence the term correlative microscopy."
Watching how hydrogen oxidizes to water
Thus, it became possible to study the oxidation of hydrogen on structurally well-defined microscopic regions of a rhodium foil (structure determination by researchers at USTEM of TU Wien) in a versatile way and in real time.
The reaction spreads over the surface like a wave, revealing a new kind of pattern formation that had never been encountered before. "In front of the spreading reaction front, new small islands of catalytically active areas form, accelerating the propagation of the reaction," says Prof. Rupprechter. In computer simulations that provide virtual reaction microscopy, the team was able to model and explain the formation of these islands.
Through the correlative approach, it was now possible to effectively use the specific strength of each of the respective microscopy methods (spatial and energy resolution, field of view, magnification down to the nanometer range), and thus to image an ongoing catalytic reaction in unprecedented detail.
The oxidation of hydrogen to water by solid catalysts is one of the significant processes that enable energy generation without combustion and without pollution (the exhaust gas consists of pure water), e.g., in fuel cells. For future developments of new green energy production technologies, it will be important to watch ongoing catalytic reactions at work with several eyes in order to deeply understand fine details of the catalytic processes.
The research was published in ACS Catalysis.
More information: Johannes Zeininger et al, Pattern Formation in Catalytic H2 Oxidation on Rh: Zooming in by Correlative Microscopy, ACS Catalysis (2022). DOI: 10.1021/acscatal.2c03692
Journal information: ACS Catalysis
Provided by Vienna University of Technology