Dense and permeable: Molecular organization of tight junctions decoded
They seal epithelial cells and, under certain conditions, allow the passage of ions and water: Tight junctions form a paracellular barrier in tissues and their dysfunction is associated with diseases. Although their molecular components have been known since the 1990s, it is not apparent how the 26 proteins called claudins are organized.
Scientists from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) have now gained deep insights into the structure of tight junctions, using super-resolution stimulated emission depletion (STED) microscopy. It is the first time that the basic mechanism underlying all epithelial barrier properties has been described.
Tight junctions (TJ) are normally excellent at enabling the passage of necessary ions or molecules, while forming a dense barrier to prevent unwanted bacteria and their toxins from entering the body. These paracellular barriers, which can simultaneously be selective ion and water channels, are found wherever epithelial cells or endothelial cells meet, i.e. where different tissues are connected to each other or when the lumen of an organ needs to be sealed from the blood stream.
The existence of tight junctions was discovered some 60 years ago, and their main molecular components have been known for 30 years: 26 membrane proteins called claudins. Depending on the cell, claudins are organized in various constellations to form semi-permeable meshworks up to several hundred nanometers wide. Usually, multiple claudins come together, but some barriers consist of just one or two structural proteins.
But the question is, how are claudins organized so as to create different barrier properties depending on the cell or tissue in question? And, to what extent do claudins depend on each other in the process? Until now, these questions have remained unanswered because it was impossible to see through the structure of strands, which are only about ten nanometers thick. Now scientists from the FMP have succeeded in doing just that using STED microscopy.
"This type of super-resolution microscopy and an excellent team of cell biologists, computer scientists and physiologists have helped us to shed light on the molecular architecture of tight junctions," said Dr. Martin Lehmann, head of the Cellular Imaging Group last author of the study. "We have now been able for the first time to describe the mechanism underlying the main epithelial barrier properties."
Using STED to resolve single meshworks
Normally, the resolution of fluorescence microscopes is limited to about 250 nanometers. Using STED microscopy, 50 nanometers or less are possible. This literally gave the researchers greater insight.
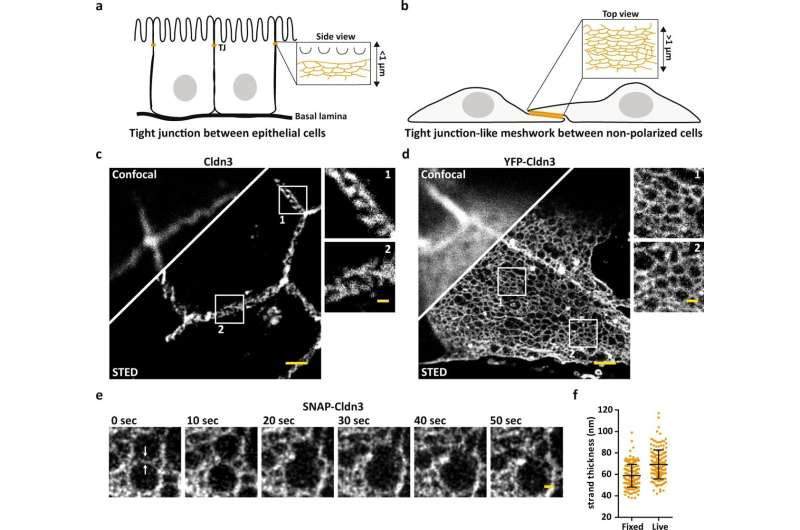
"With standard fluorescence microscopy, we would never have penetrated the dense organization of the tight junction, but STED has enabled us to resolve the individual meshes of the network. As a result, we are now able to determine the exact position of proteins, as well as to see whether claudins intermix or separate, and how they segregate," states Hannes Gonschior, the first author of the study, who conducted his Ph.D. thesis on the topic at the FMP. "This nanoscale organization was previously unknown."
First, the studies were carried out at the cellular level, and then in intestinal and kidney tissue of mice. Striking images reproduced the fluorescently labeled proteins in different colors, showing where which proteins are located and how they chain together to form a colorful zipper.
Three findings from the study, now published in Nature Communications, are of particular note:
- First: Claudins seal intercellular spaces for ions and small molecules—much like a zipper. These seals are selectively ion-permeable, depending on the tissue and composition of the tight junction.
- Second: One in two claudins are unable to polymerize into strands. They are reliant on other team members to form and "functionalize" a tight junction.
- Third: Claudins interact with each other in five organization principles. This means that there are five different ways, in which they can intermix or segregate.
Creation of model for drug discovery
The fact that FMP researchers have been able to determine the nanoscale organization of tight junctions for the first time is a major success for basic research. But medicine can also benefit from the breakthrough. This is because mutations in claudins play a role in a number of hereditary diseases, the most obvious one being the HELIX syndrome—a rare condition causing reduced sweat production.
A mutation in claudin 10b is the culprit, causing hypohidrosis, and lacrimal and salivary gland defects, as well as impaired calcium and magnesium regulation in the kidney. The team of researchers had also experimented with this disease mutant.
"Our research is still a long way from having clinical relevance," stated biophysicist, Martin Lehmann, assessing their findings. "But at least now we understand how these meshworks are structured. This is the first step, which will allow us to search for small molecules that open or close these barriers."
Cell biologist Hannes Gonschior added that they "have found a simplified model for drug discovery and, more generally, conducted research into the paracellular passage of ions. It is very likely that our findings will enable us to understand previously unexplained clinical phenotypes and symptoms—with a defect in one of these particular paracellular barriers."
More information: Hannes Gonschior et al, Nanoscale segregation of channel and barrier claudins enables paracellular ion flux, Nature Communications (2022). DOI: 10.1038/s41467-022-32533-4
Journal information: Nature Communications
Provided by Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP)