This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Finding the chink in coronavirus's armor—experiment reveals how the main protease of SARS-CoV-2 protects itself
The COVID-19 pandemic resulted in millions of deaths. Despite an unparalleled collaborative research effort that led to effective vaccines and therapies being produced in record-breaking time, a complete understanding of the structure and lifecycle of the coronavirus known as SARS-CoV-2 is still lacking.
Scientists used the biolabs and the SPB/SFX instrument at the European XFEL to study the main protease, or Mpro, of the virus to understand how it protects itself from oxidative damage. The results add key knowledge to our understanding of the workings of SARS-CoV-2 and the field of viral biology.
The paper is published in the journal Nature Communications.
Between January 2020 and March 2023, over six million people died as a result of the respiratory disease COVID-19, and several hundred million were infected. The disease is caused by SARS-CoV-2, a coronavirus.
"Coronaviruses are a group of RNA viruses that cause illnesses and diseases in mammals and birds," explains European XFEL scientist Richard Bean. "However, despite their significant relevance for global human health, there is still a lot to learn about the structure and function of coronaviruses in general and SARS-CoV-2 in particular."
In response to the outbreak of the pandemic, scientists and scientific organizations around the globe poured efforts into studying the structure, dynamics, and function of SARS-CoV-2 in search of vaccines and therapies. Due to its central role in the replication cycle of the virus, the main protease—an enzyme that liberates newly made pieces of the virus from one another—soon emerged as a key antiviral drug target.
The main protease, or Mpro, is particularly attractive for drug development because it plays a central role in viral replication, and also because it is quite different from all human proteins. This allows therapies to specifically target the virus while minimizing side effects that might harm patients. Previous drug discovery programs targeting other viruses have succeeded using viral protease inhibitors, making a successful outcome in the case of SARS-CoV-2 more likely.
"While the height of the COVID-19 pandemic may have passed, there is still a lot of value in studying the SARS-CoV-2 virus," says Thomas Lane from the Center for Free-Electron Laser Science (CFEL) in Hamburg. "COVID continues to present a significant health threat worldwide. Given the persistence of this virus and the possible emergence of future pathogenic coronaviruses, it is imperative we develop a deeper understanding of Mpro and its role in viral function."
In a recent experiment at the SPB/SFX instrument at the European XFEL, Lane and colleagues used the intense X-ray beam to study Mpro. Several previous structural studies focusing on Mpro have highlighted a number of peculiarities.
"Firstly, the protein forms a 3D structure known as a dimer when it is found in high concentrations," explains European XFEL scientist Robin Schubert, who was involved in the experiment. "This structural habit seems to directly influence its activity—but we don't know precisely why this is important for the virus." Alongside key insights into the 3D structure, recent studies have also hinted at the importance of cellular oxygen levels for protease activity.
"It seems that even mild exposure to oxygen decreases Mpro's activity," explains Patrick Reinke, also from CFEL. Indeed, in the presence of sufficient oxygen, turnover ceases altogether. But this process is reversible—if the oxygen is removed, the enzyme reactivates itself, suggesting the system has evolved protective mechanisms to survive in an oxidative environment.
"Oxidative stress has been shown to regulate the function of other viruses, such as HIV," Reinke adds. "It has been suggested that structural changes in the protease let it escape oxidative damage in oxygen-rich environments. However, we're still unsure of how these protective mechanisms impact viral fitness."
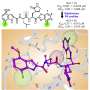
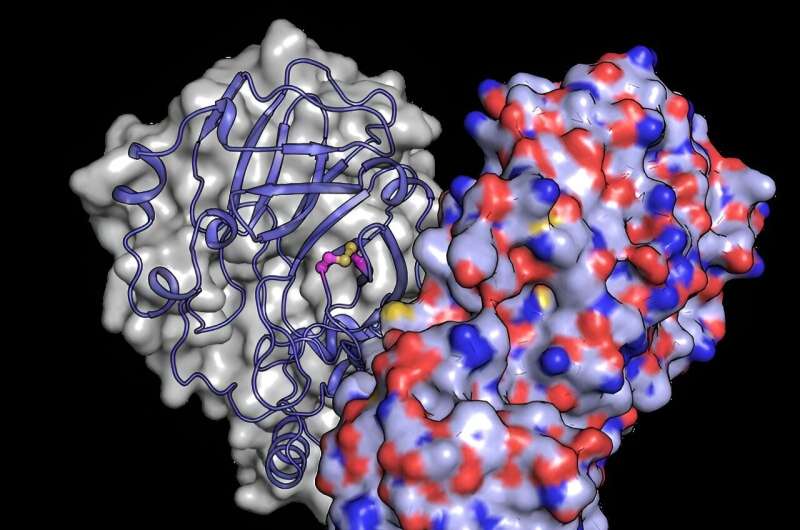
To better understand how structural changes protect the protein from oxygen damage, the team used the European XFEL's powerful X-ray beam to reveal the structure of Mpro after it had been exposed to oxygen. They discovered a structural rearrangement of Mpro in which a bond forms between two cysteine residues: the active site cysteine C145 and a distal cysteine C117.
To accomplish this, the team produced large amounts of Mpro over the course of several months in the biolabs at European XFEL and turned it into microcrystals, some of which were grown in the presence of oxygen. Finally, the microcrystals were sent flying in front of the European XFEL beam at the SPB/SFX instrument using a liquid jet.
Such small crystals are impossible to study using traditional light sources because the amount of radiation needed to generate enough data from the crystals would destroy them. The X-rays pulses produced by the European XFEL, however, are so powerful and short that they can be used to capture an image of the protein crystal before it has time to disintegrate.
"Our results show that the active site cysteine, which conducts the enzyme's chemistry, can sneakily hide itself from oxidative damage," says Schubert. Typically, oxidation can irreversibly damage cysteines.
Upon oxidation, however, Mpro protects its most important cysteine by forming what is known as a "disulfide bond," which buries it in the core of the protein structure. Then, if moved back into a safe, low-oxygen environment, the disulfide bond can break, revealing the active cysteine, which resumes its original function.
"The experiments performed at the European XFEL reveal a picture of the protein in its hidden disulfide state, confirming it exists and uncovering how it works," says Schubert.
"Mpro exhibits an unusually rich set of oxidation modifications, and our experiment adds a key piece to that story," says Lane. The scientists are excited about what their data indicate and about their next steps. "Mpro is a linchpin of coronavirus biology and the premier target for anti-COVID-19 small-molecule therapeutics.
"The enzyme's function has been shown to be regulated via both dimerization and oxidation, and it's clear that these regulatory mechanisms are biophysically correlated. While our structures provide mechanistic insight into these properties of Mpro, we must now understand how regulation based on oxidative stress or protein concentration impact viral fitness. This will provide deeper insight into viral biology and hopefully open new opportunities to disrupt that biology with life-preserving medicines."
More information: Patrick Y. A. Reinke et al, SARS-CoV-2 Mpro responds to oxidation by forming disulfide and NOS/SONOS bonds, Nature Communications (2024). DOI: 10.1038/s41467-024-48109-3
Journal information: Nature Communications
Provided by European XFEL