This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study reveals link between selenium and COVID-19 severity
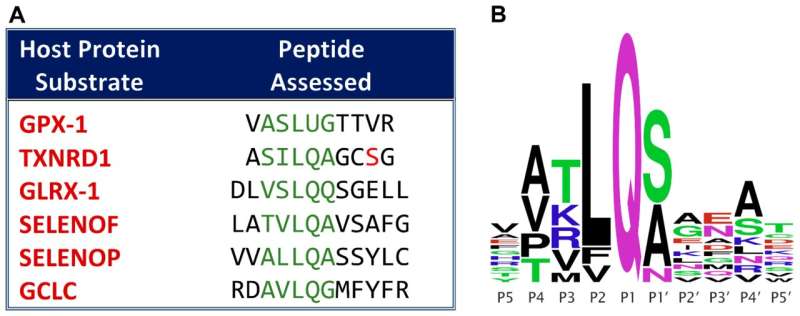
Chemists from the University of North Carolina at Greensboro released a study in the journal Antioxidants revealing a new basis for the link between dietary selenium and COVID-19 severity. Building on a previous study that identified a set of six host proteins potentially targeted by SARS-CoV-2, their study confirmed cleavage of three previously predicted protein target sites from selenoproteins, which have antioxidant and antiviral defensive roles.
In addition, they discovered that the viral protease targets and potentially inactivates the rate-limiting enzyme in glutathione synthesis, glutamate-cysteine ligase catalytic subunit (GCLC). Their findings indicate that COVID destroys the antioxidant activity of a key human selenoprotein and reveals a basis for reduced glutathione levels that are seen in COVID-19 patients, which weakens immunity and enables the virus to replicate more efficiently.
"This is the first time that a study has shown a virus to have an active strategy to destroy human selenium-containing proteins," said Ethan W. Taylor, professor of biomedical chemistry at UNCG and one of the authors of the study. "Selenium biochemistry may be a key in understanding how to strengthen the immune system against COVID."
The authors of the study–Ignacio A. Gallardo, Daniel A. Todd, Stella T. Lima, Jonathan R. Checkan, Norman H. Chu, and Taylor, all researchers in the Department of Chemistry and Biochemistry–built on previous research that established the association between dietary selenium levels and the clinical outcome of viral infections.
Following up on a 2020 paper from the Taylor group that identified six host proteins that contain potential SARS-CoV-2 main protease (Mpro) cleavage sites, they demonstrated experimentally that Mpro can cleave four of the six predicted target sites, including those from three selenoproteins: thioredoxin reductase 1 (TXNRD1), selenoprotein F and selenoprotein P, as well as the rate-limiting enzyme in glutathione synthesis, glutamate-cysteine ligase catalytic subunit (GCLC).
The cloned full length TXNRD1 protein was cleaved by SARS-CoV-2 Mpro in a manner that destroyed its antioxidant activity, by cutting off its C-terminal redox center.
"The class of proteins targeted by the virus for proteolysis have antioxidant and antiviral defensive roles, as they can disrupt the structure of viral proteins via disulfide bond reduction," said Taylor. "Thus, viral infectivity could be enhanced by knockdown of several of these proteins."
Proteolytic knockdown of both TXNRD1 and GCLC is also consistent with a viral strategy to inhibit DNA synthesis, conserving the pool of ribonucleotides for increased RNA synthesis and virus production.
These research findings suggest a need for further investigation of the clinical potential not only of selenium supplementation, but also of various dietary sulfur compounds that are precursors of glutathione. These could include the well known supplement N-acetyl cysteine (NAC), the combination of NAC plus glycine, or gamma-glutamyl cysteine (GGC, the product of the GCLC enzyme that the virus is trying to destroy).
Alternatively, the glutathione mimic alpha lipoic acid (ALA) can substitute in many of the roles of glutathione itself. Further investigation could lead to new complementary symptomatic treatments for COVID patients.
More information: Ignacio A. Gallardo et al, SARS-CoV-2 Main Protease Targets Host Selenoproteins and Glutathione Biosynthesis for Knockdown via Proteolysis, Potentially Disrupting the Thioredoxin and Glutaredoxin Redox Cycles, Antioxidants (2023). DOI: 10.3390/antiox12030559
Journal information: Antioxidants
Provided by University of North Carolina at Greensboro