A general chemical principle for creating closure-stabilizing integrin inhibitors
Name a biological function, and proteins called integrins are probably involved in it.
Together, the 24 members of the integrin family allow cells to attach to one another and to the matrix that surrounds them. They help cells decide what to become, where to go, how to respond to their environments, and when to grow, divide, or die.
Integrins' ubiquity and versatility also mean that when cells bearing them go awry, these proteins can contribute to a range of diseases, from autoimmune diseases to cancer.
The FDA has so far approved six drugs that reduce the activity of specific integrins to treat illnesses such as multiple sclerosis and ulcerative colitis and to prevent blood clots from forming. To the disappointment of scientists, doctors, and patients, however, other promising candidates have failed in clinical trials and curtailed integrins' potential as treatment targets.
New work led by researchers at Harvard Medical School and Boston Children's Hospital uncovers a reason for the failures—and offers a potential solution.
Taking a close look at an integrin involved in blood clotting, Timothy Springer, the Latham Family Professor of Biological Chemistry and Molecular Pharmacology at HMS and Boston Children's, and colleagues found that failed drugs for two different integrins inadvertently encourage the integrins to open up into their "on" position, potentially driving integrin activity instead of quelling it.
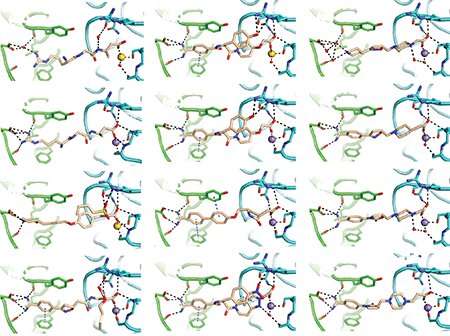
The team revealed that in its closed or "off" position, the integrin contains a water molecule held in place by a series of chemical bonds. The integrin ejects the water molecule when activated.
Once they learned what was happening, the researchers were able to design integrin blockers that coaxed the clotting protein into its "off" position by holding the water molecule in place with a nitrogen atom.
Further tests hinted that water molecules play the same role in other integrins, indicating that the team's strategy could work more broadly.
The findings, published in the journal Cell on Sept. 15, forge a clearer path for drug development and deepen researchers' understanding of how integrins work normally.
"The same water-harnessing design principle has already been extended to another integrin, and structural information suggests that researchers can design drugs to target further members of the integrin family to treat diseases that cause great suffering," said Springer, who is a member of the Program in Cellular and Molecular Medicine at Boston Children's.
"It's always gratifying to work on a project that is both scientifically and medically important," he added.
More information: Fu-Yang Lin et al, A general chemical principle for creating closure-stabilizing integrin inhibitors, Cell (2022). DOI: 10.1016/j.cell.2022.08.008
Journal information: Cell
Provided by Harvard Medical School





















