Molecule from ancient bacteria-like cells may shed new light on sexual reproduction
A study presents the theory that egg-sperm fusion, a crucial feature of sexual reproduction in plants and animals, may have originated from an ancient form of genetic exchange that involved the fusion of bacteria-like microorganisms called archaea. The results, published in Nature Communications, may open an entirely new perspective on the evolution of sex.
"Archaeal proteins with membrane fusion activity might help us to understand how cells evolved from apparently simple forms sharing discrete pieces of DNA to today's complex life forms undergoing sexual reproduction," explains Shunsuke Nishio, researcher at the Department of Biosciences and Nutrition, Karolinska Institutet, and one of the study's first authors.
The fusion of egg and sperm, specialized cells that carry the genetic information for the next generation, is the climax of sexual reproduction. Because uncontrolled cell fusion is lethal, plants and animals use special proteins called fusogens to control when and where this process takes place.
Evolutionary insight from an important ancestral molecule
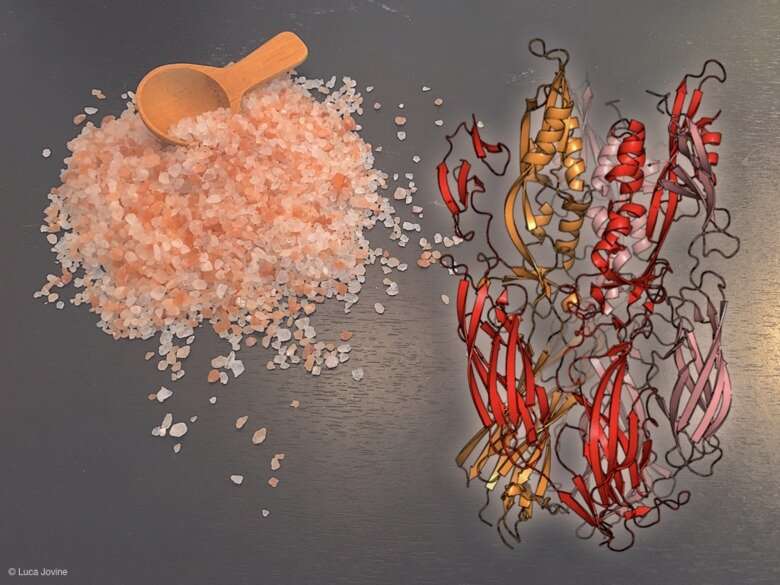
The new study reports that archaea, bacteria-like cells believed to have originated more than 3 billion years ago, can contain a protein (Fusexin 1 or Fsx1) that resembles a type of fusogen (HAP2) that had previously been identified in viruses, plants and invertebrate animals.
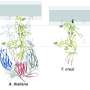
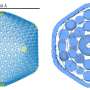
The researchers combined computational evolutionary biology, AlphaFold-based protein modeling, X-ray crystallography, and functional studies to show that the archaeal protein Fsx1 is a bona fide fusogen. This is both because it is structurally similar to the previously identified HAP2 fusogen and able to promote cell-cell fusion when expressed in other cell types.
"Gamete fusion has fascinated mankind for more than 150 years. Since we already knew that HAP2-like proteins are used to fuse the membrane of enveloped viruses (such as zika, dengue and rubella) with host cells, we wondered whether this key molecule originated in a virus and was then repurposed for gamete fusion in plants and animals or the other way around," says Luca Jovine, professor at the Department of Biosciences and Nutrition, Karolinska Institutet, and one of the study's corresponding authors.
"The discovery that ancient creatures like archaea can also contain a HAP2-like protein now raises a third intriguing possibility whereby Fusexin1 is the ancestral molecule from which viral, plant, and invertebrate animal fusogens derive."
The origin of sex
The study was an international collaboration with academic research groups from Israel, Argentina, Uruguay, and Switzerland, as well as the European Synchrotron Radiation Facility (ESRF) in France and the UK-based AI company DeepMind that developed AlphaFold.
The next step will be to work out what Fsx1 proteins are doing in nature, for example, if they fuse archaeal cells—like their plant and animal HAP2 counterparts fuse gametes—to promote a sex-like DNA exchange. Parallel studies will also be needed to accurately chart the evolutionary history connecting Fsx1 and HAP2 in order to firmly establish their origin.
More information: David Moi et al, Discovery of archaeal fusexins homologous to eukaryotic HAP2/GCS1 gamete fusion proteins, Nature Communications (2022). DOI: 10.1038/s41467-022-31564-1
Journal information: Nature Communications
Provided by Karolinska Institutet