Focusing on the oxygen reduction reaction in the search for more efficient hydrogen cells

A tenacious postdoc researcher persuaded Professor Marc Koper to research the oxygen reduction reaction. In Koper's eyes, there was little of interest there. But they promptly discovered a whole new way to improve fuel cells on hydrogen and oxygen. Their article appeared in Nature Catalysis on 7 July.
There are few hydrogen-powered cars driving around the Netherlands: one of them, a Toyota Mirai, is owned by Shell Nederland director Marjan van Loon. Marc Koper, Professor of Catalysis and Surface Chemistry, said: "She is one of the few who can actually fill up with hydrogen in the Netherlands, at Shell in Amsterdam."
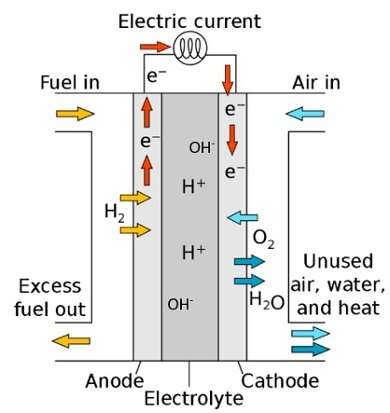
Toyota is working on better, more efficient fuel cells, in order to be able to introduce hydrogen driving on a large scale. But the technology has not advanced that far yet, as there are two major problems. First, too much of the rare metal platinum is needed in the fuel cells. Second, the part of the reaction that converts oxygen into water, the so-called oxygen reduction reaction, has to become more efficient.
Weaker oxygen bonding was thought to be the only knob to turn
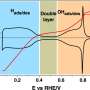
For years, researchers have thought there is only one way to make oxygen reduction more efficient. In the fuel cell with two poles—the anode and the cathode—that reaction takes place on the cathode on which there are many small platinum particles. Oxygen breaks down there into oxygen atoms bound to the platinum. These atoms then react further to form the final product, water.
Koper: "The current theory is that we have to look for a cathode that holds the oxygen atoms a little less strongly. And also, that this is the only knob you can turn to make the oxygen reduction easier. Toyota uses a cathode that contains platinum and some cobalt. This cobalt helps to weaken the binding of oxygen to platinum. So that theory works well."
Koper is one of the most cited scientists in the world, received many grants and was awarded the largest and most prestigious Dutch science prize in 2021. He received the Spinoza Prize of 2.5 million euros partly because his research can contribute to the energy transition. He investigates how electrical energy can help make or break chemical compounds. This would allow you to store green power, so that you can save it for when the sun does not shine or the wind dies.
But that is not what Koper is interested in
Koper is honest about his motives: they are not related to world improvement. "I want to understand at an atomic level what happens when you send electricity through an electrochemical cell," he said in an interview on the occasion of his Spinoza Prize. The search for a better cathode to bind oxygen less strongly is not so exciting to him, however important the problem is. "In my opinion, there is not much more to be gained scientifically from fine tuning the cathode with the best ratio of cobalt to platinum."
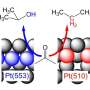
But then postdoc Mingchuan Luo came to work for Koper. "He insisted on working on oxygen reduction." Koper then proposed to find out what happens if you play with the composition of the electrolyte, the medium that separates the anode from the cathode. The electrolyte contains a certain concentration of negatively charged ions: anions. Luo experimented with different concentrations of anions.
They discovered a new knob to turn
Koper: "We then discovered that the reduction of oxygen sometimes goes faster than expected, even though the binding of oxygen to the cathode seems stronger. The prevailing idea that you can only achieve more efficient oxygen reduction with weaker oxygen bonding is therefore incorrect. It seems that those anions in the electrolyte influence another process in the oxygen reduction reaction. Namely: the ease with which those platinum bound oxygen atoms are converted to hydroxide (OH-), the last step before making water. This gives us a new knob to turn, which is fundamentally different from the usual one."
So instead of just one route to more efficient hydrogen cells, there are at least two. This is very interesting to Koper, who mainly wants to understand what happens at the molecular level and why these anions play such an important role. His colleagues at Toyota will also find it interesting, though they cannot make great progress with it right away. "At the moment, this new insight mainly raises new questions. We now have to go and find out exactly what is going on."
More information: Mingchuan Luo et al, A kinetic descriptor for the electrolyte effect on the oxygen reduction kinetics on Pt(111), Nature Catalysis (2022). DOI: 10.1038/s41929-022-00810-6
Journal information: Nature Catalysis
Provided by Leiden University