Inside the jellyfish's sting: Exploring the micro-architecture of a cellular weapon
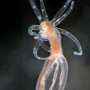
Summertime beachgoers are all too familiar with the painful reality of a jellyfish sting. But how do the stinging cells of jellyfish and their coral and sea anemone cousins actually work? New research from the Stowers Institute for Medical Research unveils a precise operational model for the stinging organelle of the starlet sea anemone, Nematostella vectensis. The study, published online in Nature Communications on June 17, 2022, was led by Ahmet Karabulut, a predoctoral researcher in the lab of Matt Gibson, Ph.D. Their work involved the application of cutting-edge microscopic imaging technologies along with the development of a biophysical model to enable a comprehensive understanding of a mechanism that has remained elusive for over a century. Insights from the work could lead to beneficial applications in medicine, including the development of microscopic therapeutic delivery devices for humans.
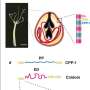
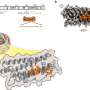
The Stowers team's new model for stinging cell function provides crucial insights into the extraordinarily complex architecture and firing mechanism of nematocysts, the technical name for cnidarian stinging organelles. Karabulut and Gibson, in collaboration with scientists at the Stowers Institute Technology Centers, used advanced imaging, three-dimensional electron microscopy, and gene knockdown approaches to discover that the kinetic energy required for piercing and poisoning a target involved both osmotic pressure and elastic energy stored within multiple nematocyst sub-structures.
"We utilized fluorescence microscopy, advanced imaging techniques and 3D electron microscopy combined with genetic perturbations to understand the structure and operating mechanism of nematocysts," said Karabulut.
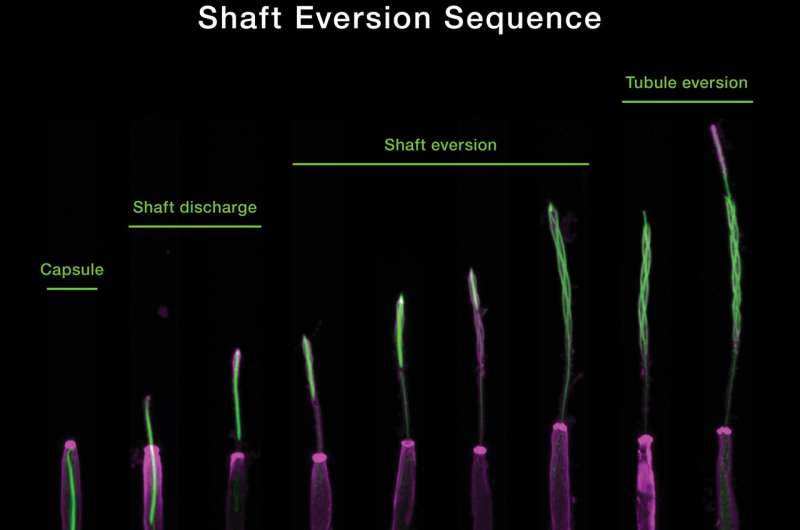
Using these state-of-the-art methods, the researchers characterized the explosive discharge and biomechanical transformation of N. vectensis nematocysts during firing, grouping this process into three distinct phases. The first phase is the initial, projectile-like discharge and target penetration of a densely coiled thread from the nematocyst capsule. This process is driven by a change in osmotic pressure from the sudden influx of water and elastic stretching of the capsule. The second phase marks the discharge and elongation of the thread's shaft substructure which is further propelled by the release of elastic energy through a process called eversion—the mechanism where the shaft turns inside out—forming a triple helical structure to surround a fragile inner tubule decorated with barbs containing a cocktail of toxins. In the third phase, the tubule then begins its own eversion process to elongate into the soft tissue of the target, releasing neurotoxins along the way.
"Understanding this complex stinging mechanism can have potential future applications for humans," said Gibson. "This could lead to the development of new therapeutic or targeted delivery methods of medicines as well as the design of microscopic devices."
The entire stinging operation is completed within just a few thousandths of a second, making it one of the fastest biological processes occurring in nature. "The earliest phase of the firing of the nematocyst is extremely fast and hard to capture in detail," said Karabulut.
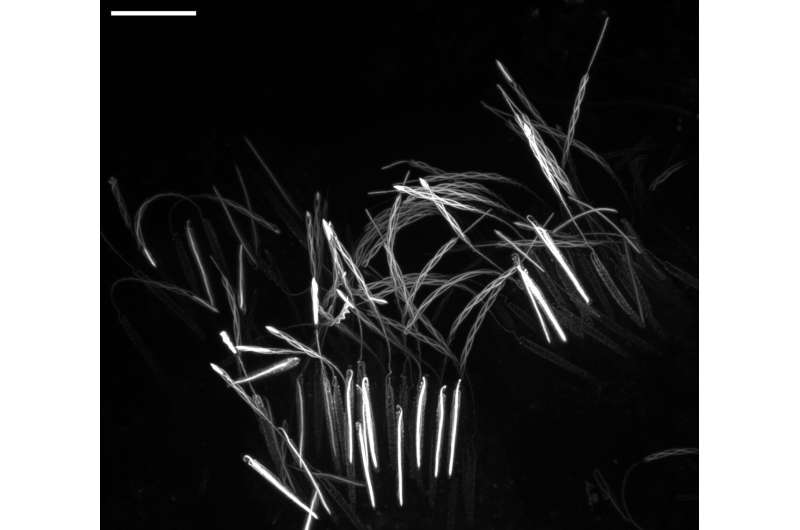
As is often the case in fundamental biological research, the initial discovery was an accident of curiosity. Karabulut incorporated fluorescent dye into a sea anemone to see what it looked like when the nematocyst-rich tentacles were triggered. After applying a combination of solutions to both activate nematocyst discharge and simultaneously preserve their delicate substructures in time and space, he was shocked to have serendipitously captured multiple nematocysts at different stages of firing.
"Under the microscope, I saw a stunning snapshot of discharging threads on a tentacle. It was like a fireworks show. I realized nematocysts partially discharged their threads while the reagent I used simultaneously and instantaneously fixed the samples," said Karabulut.
"I was able to capture images that showed the geometric transformations of the thread during firing in a beautifully orchestrated process," said Karabulut. "After further examination, we were able to fully comprehend the geometric transformations of the nematocyst thread during its operation."
Elucidating the elaborate choreography of nematocyst firing in a sea anemone has some interesting implications for the design of engineered microscopic devices, and this collaborative effort between the Gibson Lab and the Stowers Institute Technology Centers may have future applications for delivering medicines in humans at the cellular level.
Coauthors include Melainia McClain, Boris Rubinstein, Ph.D., Keith Z. Sabin, Ph.D., and Sean McKinney, Ph.D.
More information: Ahmet Karabulut et al, The architecture and operating mechanism of a cnidarian stinging organelle, Nature Communications (2022). DOI: 10.1038/s41467-022-31090-0
Journal information: Nature Communications
Provided by Stowers Institute for Medical Research