Researchers demonstrate enzyme-MOF flow reactor
Enzymes are considered true multi-functional talents. They accelerate many chemical reactions as catalysts. In organisms, they are involved in vital processes. In technology and industry, they can be used as tools of white biotechnology and help save energy and resources. Enzymes in detergents, for instance, detach dirt at low temperatures already. Enzymes are also applied in environmental technology, food processing, medicine production, medical diagnostics, and many other branches.
Enzymes mostly represent proteins. To use the potential of these huge molecules in so-called cell-free biotechnology, they have to be stabilized and integrated in efficient reactor systems. This has already been achieved quite often in aqueous solvents. In organic solvents, by contrast, destabilization or denaturation occurs and catalytic properties are lost.
Researchers from KIT's Institute of Functional Interfaces (IFG), Institute of Nanotechnology, and Institute for Biological Interfaces 1—Biomolecular Micro- and Nanostructures have now succeeded in stabilizing enzymes such that they can be used in both aqueous and organic solvents. For the first time, they demonstrated a continuous enzyme reactor system of high productivity and stability. This is reported in the journal Angewandte Chemie.
MOFs facilitate separation of catalysts and products
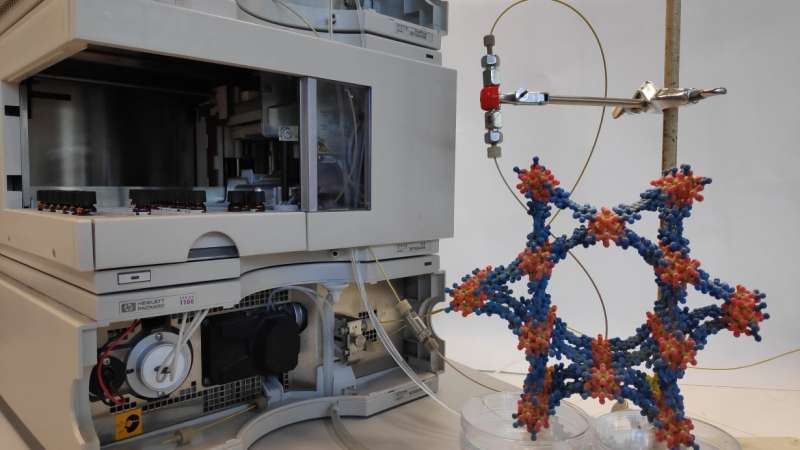
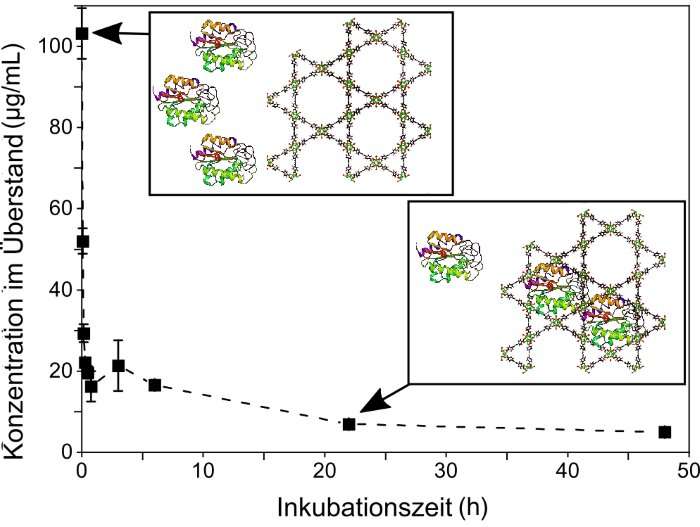
The innovative reactor system is based on metal-organic frameworks (MOFs). These consist of metallic nodes and organic connecting rods and have crystalline structures with defined pore sizes. Using different combinations of metal building blocks and organic ligands as well as various pore sizes, MOFs can be customized for various applications. In the case of cell-free use, enzymes act like cages. "We make the enzymes diffuse individually into the pores of the MOFs, that is they enter the cage voluntarily," says Professor Christof Wöll, Head of IFG. "The MOFs act as an armor and protect the sensitive biomolecules from denaturation." Thanks to the porosity of the MOFs, transport of reactants, i.e. of the substances consumed and products resulting during the reactions, can be controlled better. Moreover, MOFs facilitate complex separation of catalyst and products, says Professor Matthias Franzreb, Deputy Head of IFG and co-author of the study.
KIT researchers demonstrated time- and cost-efficient manufacture of an enzyme-MOF flow reactor. Stability of the immobilized enzyme was about 30 times that of the free enzyme. Catalytic activity reached about 30 percent of that of a free enzmye—a rather high value in view of the deformation of the enzyme embedded in the MOF pores.
More information: Raphael Greifenstein et al, MOF‐Hosted Enzymes for Continuous Flow Catalysis in Aqueous and Organic Solvents, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202117144
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Karlsruhe Institute of Technology