Subcellular vesicles regulate 'stemness' of human neural stem cells during division
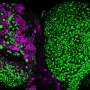
![FIG. 1. Human NSCs tend to differentiation upon FGF2 withdrawal accompanied by an increase in asymmetric LAMP1 segregation during mitosis.(A) Representative brightfield images and immunostaining of rosette-type NSCs from two healthy control cell lines (Ctrl#1 and Ctrl#2) for NSC markers (Sox2 and Nestin) and neuron markers (Tubb3 and HuC/D). (B and C) Immunostaining for Sox2 and HuC/D and respective quantification of the amount of NSCs (Sox2+) and neurons (HuC/D+) after FGF2 withdrawal from Ctrl#1-NSCs over a time course of 7 days (n = 3, two-way ANOVA with Bonferroni’s multiple comparison test, means + SEM). (D and E) Representative images of symmetric and asymmetric segregation of LAMP1+ and CD63+ vesicles during mitosis of Ctrl#1-NSCs. Yellow lines indicate ROIs surrounding daughter cells, defined by DAPI and phalloidin staining (see also fig. S1E). (F and G) Quantification of LAMP1 and CD63 asymmetry based on sum intensity ratios between paired daughter cells (see fig. S1D) in conditions with and without FGF2 (N = 6 coverslips from three independent experiments with n = 42 to 58 cells per coverslip, two-way ANOVA with Bonferroni’s multiple comparison test, means + SEM). (H) Representative images of LAMP1 costained with Notch1 receptors in different mitotic phases of Ctrl#1-NSCs. (I) Correlation of asymmetry indices A of LAMP1 and Notch1 in Ctrl#1 and Ctrl#2-NSCs. Dots represent individual mitotic event, and linear regression is shown. Scale bars, 100 μm (A and B), 10 μm (D, E, and H), 5 μm [zoomed in (H)]. DNA was counterstained with DAPI. *P < 0.05 and ***P < 0.001. n.s., not significant. See also fig. S1. Credit: DOI: 10.1126/sciadv.abl5792 Subcellular vesicles regulate 'stemness' of human neural stem cells during division](https://scx1.b-cdn.net/csz/news/800a/2022/subcellular-vesicles-r.jpg)
Stem cells are one of the few cell types within the body that have the ability to both self-renew and produce cells that develop into more specialized cell types which eventually built up a functional organ. During development, these cells face the delicate task to switch between these two functions in a timed and programmable manner. One mechanism how stem cells achieve this shift in cell fate is the asymmetric concentration of cell fate determinants during the late stages of cell division, or mitosis, generating two distinct sibling cells. The team from the Central Institute of Mental Health (CIMH) has now investigated how sibling cells with such unequal properties are generated by human neural stem cells. In the study now published in Science Advances, they describe how asymmetric inheritance of a subcellular vesicle compartment, namely lysosomes, influence the localization and activity of one important cell fate determining signaling pathway, the Notch pathway.
Notch as master regulator of cellular fates
The Notch pathway signals via the eponymous Notch receptors, which are located at the cell surface. Differential inheritance of Notch activity was already studied in the context of asymmetric neural stem cell division in lower model organisms, such as fruit flies and zebrafish. Here, Notch receptors are regulated by a variety of mechanisms including the internalization from the surface into subcellular vesicles of the dividing cell. Via these, so called endosomes, the receptors are concentrated within one of the siblings, which subsequently retains its stem cell fate through biased activation of Notch signaling. Interestingly, Notch signaling is not only involved in neurodevelopment, but regulates stem cell fate in a variety of organs and tissues, including skin, gut, and the immune system.
Notch activity is inherited via lysosomes
The researchers in Mannheim now investigated how vesicular components are inherited during mitosis of human neural stem cells. Using the induced pluripotent stem cell (iPSC)-technology they were able to study this for the first time in the context of human brain development and found that lysosomes are asymmetrically inherited by the two daughter cells. Lysosomes are a specialized subset of vesicles and are commonly considered the "cellular garbage disposal."
"This was a very interesting as unexpected result' says Professor Philipp Koch from the Hector Institute for Translational Brain Research at the Central Institute of Mental Health. "This observation motivated us to search for a biological principle behind it."
"Notch signaling was a very attractive candidate as Notch has been described as a master regulator of cellular fate decisions in many species and cellular fates' says Bettina Bohl, Ph.D. student at the Hector Institute for Translational Brain Research and first author of the study. "So far, however, the idea that lysosomes act as signaling hubs for Notch signaling during mitosis has not been described." To investigate Notch signaling in dividing human neural stem cells in more detail, Prof Koch and his team followed how Notch receptors are processed in the cells. Using live cell imaging they found that soluble ligands specific for Notch receptors are internalized upon binding and that this receptor-ligand complex is intracellularly shuffled towards lysosomes. "Usually proteins become degraded within the acidic environment of these vesicles," says Prof. Koch. "This is, however, not the case for Notch receptors in neural stem cells." On the contrary the researchers demonstrated, that Notch receptors are activated once reaching the acidic lysosomes probably through an increased activity of the Notch cleaving enzyme gamma-Secretase. To correlate this observation to gene regulation induced by Notch signaling the team developed reporter iPSCs that produce a fluorescent protein once Notch receptors are activated. Thereby, they were able to follow the gene expression of HES1, a target of Notch signaling, in living neural stem cells and demonstrate that those siblings receiving more lysosomes show more activity of HES1.
Organoids as model for human brain development
Finally, the authors used human iPSC-derived brain organoids to investigate lysosome and Notch inheritance in a three-dimensional model of human brain development. They found that the orientation of the dividing cells in relation to their surroundings, more specifically their attachment to the apical membrane, can predict the symmetric versus asymmetric distribution of lysosomes and Notch receptors. These findings are in line with studies in rodents, where the localization was also found to be decisive for cell fate of dividing neural stem cells.
"Organoids are a very fascinating tool to investigate human brain development," says CIMH-researcher Dr. Julia Ladewig whose team is focused on brain organoids in development and disease. "And this study is another vivid example for their potential."
Prof Koch also emphasizes the importance of this new human model system for future research. "Our observations widen our knowledge about the regulation of Notch signaling, but also demonstrate that we are just at the beginning to understand the complex regulatory mechanisms of such important signaling pathways. It also indicates an evolutionary development of the 'fine adjustment' of this pathway from flies and fish towards mammals and especially humans," says Koch.
More information: Bettina Bohl et al, Asymmetric Notch activity by differential inheritance of lysosomes in human neural stem cells, Science Advances (2022). DOI: 10.1126/sciadv.abl5792
Journal information: Science Advances
Provided by Zentralinstitut für Seelische Gesundheit