Structural mechanism of membrane remodeling caused by the protein MakA from Vibrio cholerae
Research performed at Umeå University has led to the discovery of a pH-induced structural mechanism of membrane remodeling caused by the protein MakA, a subunit of the recently described α-pore-forming toxin from the pathogenic bacterium Vibrio cholerae. Collaborating scientists affiliated with MIMS and UCMR publish their new findings in the journal eLife.
All types of living cells are dependent on functional membranes. Proteins that form pores in biological membranes are found in many different contexts, including some bacterial toxins that promote infections and can cause damage to target cells and organelles.
Research in the group of Professor Sun Nyunt Wai at Department of Molecular Biology resulted in the initial discovery of the protein MakA as a motility-associated secreted toxin from V. cholerae using the predatory model organism Caenorhabditis elegans and in vitro grown mammalian cell models. When MakA is secreted with two other Mak proteins, it forms the MakA/B/E tripartite pore complex in mammalian cell membranes as revealed, recently through biochemical analyses and structural characterisation using X-ray crystallography in collaboration with the research team led by Professor Karina Persson at Department of Chemistry. This is typical of the proteins known as "the α-pore-forming toxins," a superfamily of membrane pore-forming protein toxins.
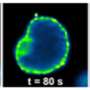
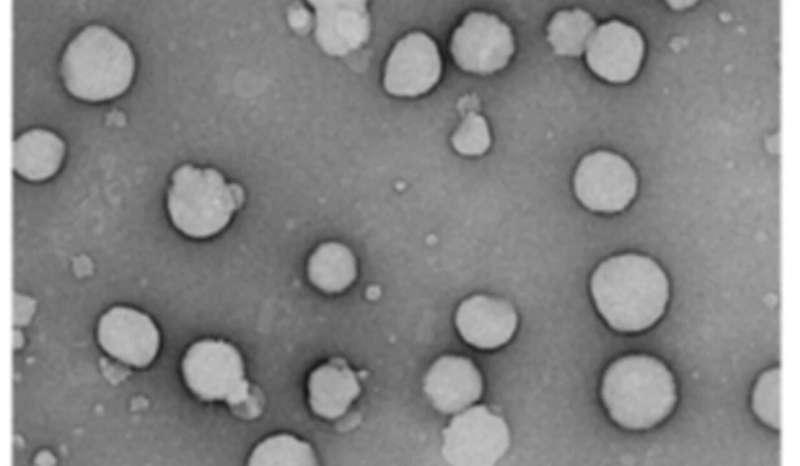
MakA alone may also attach to target cell membranes and be taken up by cultured mammalian cells via endocytosis where it accumulates in the endolysosomal membrane space. Endolysosomes are acidic organelles that break down cellular macromolecules and recycle cellular building materials. The new research work provides strong evidence for how MakA interacts with membranes under acidic conditions.
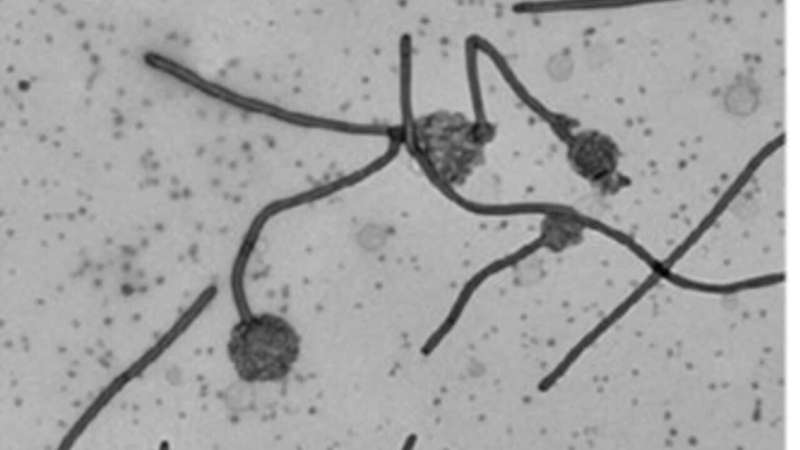
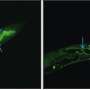
It was found that the MakA protein appears to make tube-like structures and make the acidic endolysosomal compartment leaky and not work correctly. Acidic conditions made MakA generate oligomers and changed membranes into tube-like structures, which caused cells to lose their membranes. When MakA bound to liposomes from cell lipid extracts at low pH, they formed the tube-like structures.
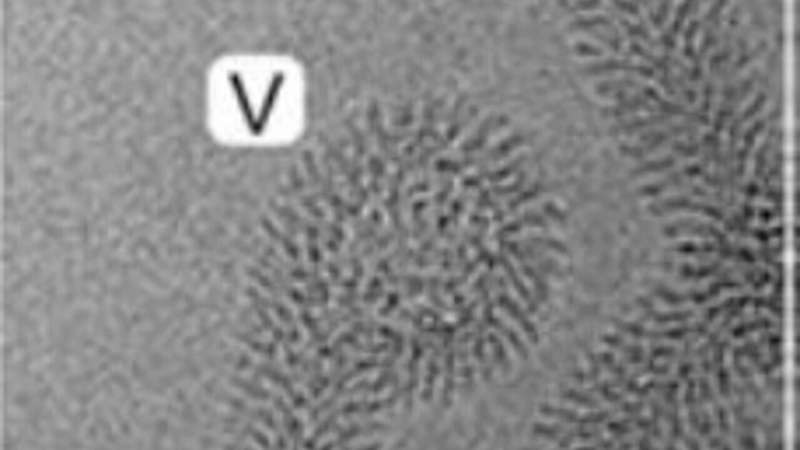
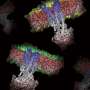
These studies unravel the dynamics of tubular growth, which occurs in a pH-, lipid-, and concentration-dependent manner. A model of the detailed protein-lipid structure was obtained by Cryo-EM analysis. The MakA action is a fascinating example of a membrane remodeling structural process triggered by pH-dependent change in the protein´s conformation. It will be of interest to a wide spectrum of scientists working on host-pathogen interactions, membrane modification, and macromolecular structure of medically significant bacterial pathogens. From the perspective of protein engineering and nanotube formation, it is suggested that the current discoveries on how MakA causes lipid/protein spiral structures might be relevant for protein/membrane engineering and synthetic biology.
More information: Aftab Nadeem et al, Protein-lipid interaction at low pH induces oligomerisation of the MakA cytotoxin from Vibrio cholerae, eLife (2022). DOI: doi.org/10.7554/eLife.73439
Journal information: eLife
Provided by Umea University