Development of a new tool which uses focused light to reduce cellular contractile force
Cell generated force plays essential roles in a wide range of biological processes, such as cell motility, cytokinesis, and tissue morphogenesis. In a study published in Nature Communications, a research team led by the Division of Quantitative Biology at the National Institute for Basic Biology in Japan has successfully developed "OptoMYPT": an optogenetic tool that utilizes focused light to reduce actin and myosin-based contractile force.
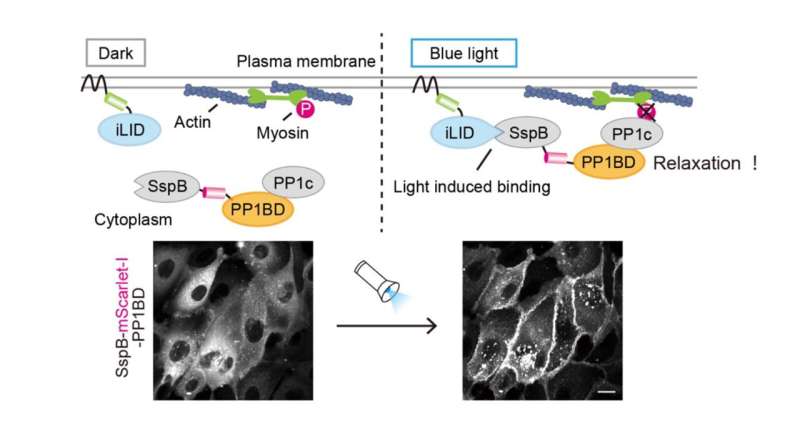
The researchers designed an optogenetic tool to directly inactivate nonmuscle myosin II, which generates cellular contractility in coordination with actin filaments. To achieve this, they focused on MYPT1, a protein required for myosin inactivation, and one that brings a phosphatase PP1c in close proximity to phosphorylated myosin. This subsequently results in dephosphorylation and inactivation of myosin. Taking advantage of this feature, the researchers aimed to optically manipulate the localization of PP1c originally existing in cells by using the PP1c binding domain (PP1BD) of MYPT1.
"To develop OptoMYPT, we used a tool called iLID to control the localization of proteins with light. This tool is based on the idea that blue light irradiation causes iLID protein to bind to SspB proteins. First, the iLID protein is localized to the cell membrane, while SspB fused with PP1BD of MYPT1 is expressed within the cytoplasm. Blue light exposure then induces the translocation of SspB-PP1BD from the cytoplasm to the membrane through binding to iLID, leading to the co-recruitment of endogenous PP1c to the membrane. Finally, the membrane-recruited PP1c dephosphorylates and inactivates myosin near the cell membrane," Kei Yamamoto, a graduate student and the lead author of this paper, explained.
As expected, PP1c was translocated to the cell membrane upon blue light illumination being exposed upon the OptoMYPT expressing cells, and consequently, actin and myosin-mediated contractile force was reduced.
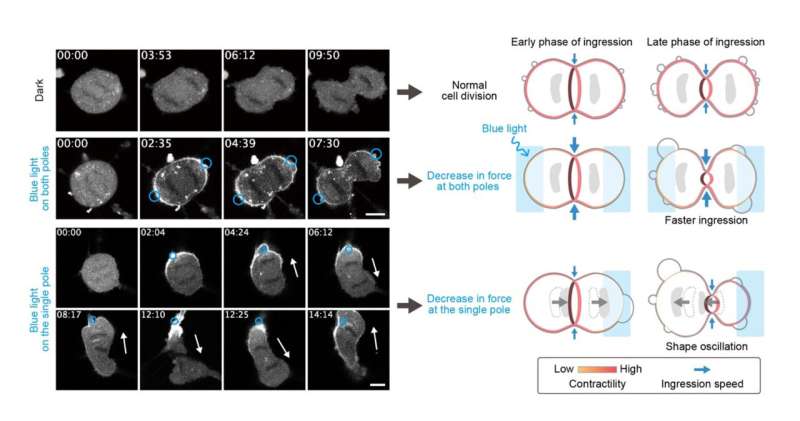
To understand the mechanics of cell division, the research team applied their OptoMYPT to dividing cells. When shining blue light upon both poles of the dividing cells to weaken the tensile force generated at the cell cortex, the ingression speed of the cell cleavage furrow was accelerated. Furthermore, when the tension of the cell cortex was weakened on only one side, an oscillatory cytoplasmic flow took place between the two daughter cells. Thus, by applying local force perturbations using OptoMYPT, the research team demonstrated that the optimal strength and symmetry of the forces generated at the cell surface are essential for the normal progression of cell division.
"We are convinced that this tool, OptoMYPT, will be useful for understanding various embryological and cell biological phenomena involving the actomyosin cytoskeleton," said Professor Kazuhiro Aoki, a member of the research team. He added that "in the future, we expect that it can be used for freely designing the shapes of cells and tissues and for forming artificial organs."
More information: Kei Yamamoto et al, Optogenetic relaxation of actomyosin contractility uncovers mechanistic roles of cortical tension during cytokinesis, Nature Communications (2021). DOI: 10.1038/s41467-021-27458-3
Journal information: Nature Communications
Provided by National Institutes of Natural Sciences