New insights into cell polarity
A previously unknown mechanism involving the protein Scribble helps maintain polarity in cells, according to a Northwestern Medicine study published in the Journal of Biological Chemistry.
This new mechanism sheds light on the complex web of systems that keep cells pointing in the correct direction, according to Sergey Troyanovsky, Ph.D., professor of Dermatology, of Cell and Developmental Biology and senior author of the study.
"This is the architecture of the cell, like how individual buildings come together to form the whole city," Troyanovsky said.
Cell polarity dictates an orientation for all cells, providing a matrix-like structure that makes up all tissue in the body. Apical and basal polarity define "up" and "down," according to Troyanovsky.
"Cells need to know which side is the head and which side is the legs, so they are correctly oriented in tissue," Troyanovsky said.
This polarity is maintained by certain proteins, which sit on the apical (top) or basal/lateral (bottom) cell membrane and signal to one another or to cell-cell junctions. However, exactly how these proteins regulate and communicate to one another was unknown.
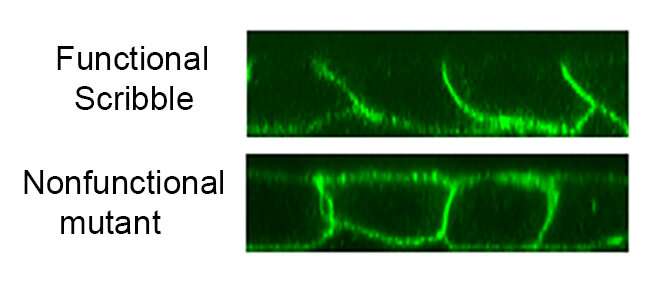
In the current study, investigators examined the protein Scribble, a well-known protein involved in development of the lateral plasma membrane. About one-third of this protein is sufficient to maintain correct polarity of the cells, but the mechanism of this activity was unclear, Troyanovsky said.
Searching for this mechanism, the scientists measured the breadth of protein interactors on the cell membrane that are present in the functional Scribble and its mutant that is unable to maintain polarity. The difference in interactors between these two proteins is just five protein interactors, according to the study. One of these protein is the well-known protein phosphatase 1 (PP1).
Within Scribble, these five proteins use the same binding interface—the same amino acids—so Scribble cannot interact with all interactors simultaneously.
"It only picks one," Troyanovsky said.
Mutual exclusivity of these interactions helps Scribble function like a switch, specifically for PP1 release. As an enzyme important for activating proteins, PP1 bound to Scribble is maintained in an inactive state. However, when any of other remaining four proteins (which all are also known to regulate polarity) bind to Scribble, active PP1 is released to specific sites on the cell membrane to enact a dephosphorylation of specific targets.
Phosphorylation is an important regulator of protein function, so Troyanovsky and his collaborators are now examining the downstream impact of this released PP1, he said, searching for its importance in cell polarity.
More information: Regina B. Troyanovsky et al, Basolateral protein Scribble binds phosphatase PP1 to establish a signaling network maintaining apicobasal polarity, Journal of Biological Chemistry (2021). DOI: 10.1016/j.jbc.2021.101289
Journal information: Journal of Biological Chemistry
Provided by Northwestern University