Appearance of cystic fibrosis at the molecular scale
Despite remarkable medical advances over the last years, cystic fibrosis remains the most prevalent lethal genetic disease. It is due to mutations in the CFTR protein which is normally required to maintain proper fluid balances in key organs such as lungs, pancreas or the digestive system.
In most cases, the causative mutation, called F508del, involves only one of the 1.480 amino acids that make up the CFTR protein. This apparently minor change leads to strong deleterious effects in the protein, which becomes unable to perform its normal biological function, leading to the emergence of the disease. For years, researchers have attempted to understand how and why a simple mutation triggers such large effects on the protein structure and function, with dramatic consequences for the patients.
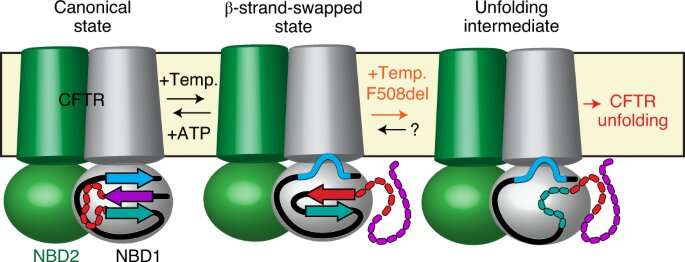
Using a combination of cutting-edge methods such as single-molecule fluorescence, X-ray crystallography, hydrogen-deuterium exchange and single molecule electrophysiology, researchers led by Cédric Govaerts—Laboratoire Structure & Fonction des membranes biologiques, Faculté des Sciences, Université libre de Bruxelles—have uncovered a completely new phenomenon in CFTR: while proteins are expected to adopt a single conformation that enables a single biological function, they have observed a new conformation of CFTR.
This structure had not been observed before and demonstrates that CFTR is not a fixed molecule but can alternate between (at least) two different conformations with potentially different functions.
Astonishingly, researchers have also observed that the most prevalent mutation, F508del, does not affect the structures themselves, but rather the transitions between them. In other words, they propose that disease-causing mutations such as F508del may not, like previously believed, perturb the final conformation of the protein but, rather the dynamics, specifically the ability of the protein to exchange between different states within the cell.
This observation changes our understanding of CFTR biology and of cystic fibrosis. In addition, his phenomenon could apply to other proteins and thus allow to understand other genetic diseases.
More information: Daniel Scholl et al, A topological switch in CFTR modulates channel activity and sensitivity to unfolding, Nature Chemical Biology (2021). DOI: 10.1038/s41589-021-00844-0
Journal information: Nature Chemical Biology
Provided by Université libre de Bruxelles