World's first 'green' synthesis of plastics from CO2
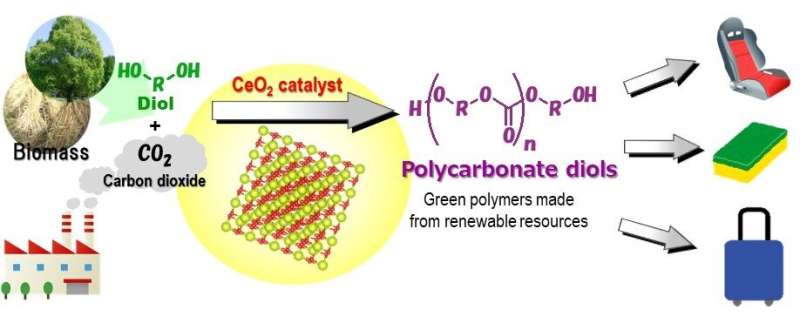
By combining a CeO2 catalyst with atmospheric carbon dioxide, researchers from Osaka City University, Tohoku University, and Nippon Steel Corporation have developed an effective catalytic process for the direct synthesis of polycarbonate diols without using dehydrating agents. Their method, published in Green Chemistry, does not rely on toxic chemical feedstock like phosgene and carbon monoxide, making it the world's first high yield "green" reaction system.
There is a worldwide need to reduce carbon dioxide, one of the major greenhouse gases, and converting it into a useful chemical compound has attracted much attention in recent years. Various effective catalyst systems have been developed but they rely on toxic chemicals that churn out unmanageable by-products. Processes using substrates that are easily available and safe, with water as the only by-product, have emerged as an alternative. Yet, high levels of water by-product keep these processes from synthesizing enough polycarbonates.
"Most processes use a dehydrating agent to keep water levels low to overcome an equilibrium," said Masazumi Tamura of the Osaka City University, "but some of the issues to address are the high pressure of carbon dioxide needed, the recovery and regeneration of the dehydrating agent, and contamination of by-products generated by its use."
To bypass these issues, the research team developed a catalytic process that does not use a dehydrating agent. By focusing on the difference in boiling points between the chemical product/diol and water, the research team predicted a high carbon fixation yield by blowing in CO2 at atmospheric pressure to evaporate excess water.
"It became clear that among the metal oxide catalysts we used," stated Keiichi Tomishige of Tohoku University, "CeO2 showed the highest activity." This simple catalytic reaction system is the first ever to successfully synthesize polycarbonate diols from carbon dioxide and diols at atmospheric pressure. "This process, without the need of dehydrating agents, can chemically convert carbon dioxide using any substrate with a boiling point sufficiently higher than water," concluded Kenji Nakao of Nippon Steel Corporation, "and can be applied to the synthesis of carbonates, carbamates, and ureas, which are useful additives for lithium-ion batteries and/or raw materials for polymer synthesis."
More information: Yu Gu et al, Direct synthesis of polycarbonate diols from atmospheric flow CO2 and diols without using dehydrating agents, Green Chemistry (2021). DOI: 10.1039/D1GC01172C
Journal information: Green Chemistry
Provided by Osaka City University