This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemists develop new sustainable reaction for creating unique molecular building blocks
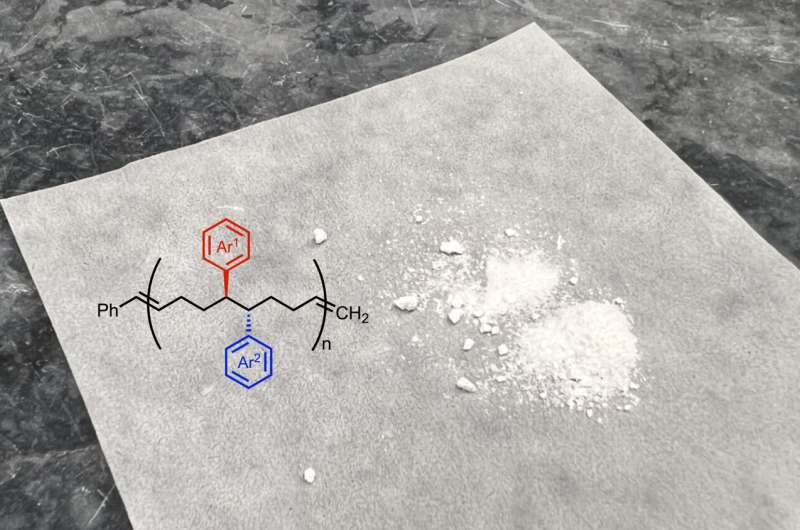
Polymers can be thought of like trains: Just as a train is composed of multiple cars, polymers are made up of multiple monomers, and the couplings between the train cars are similar to the chemical bonds that link monomers together. While polymers have myriad applications—from drug delivery to construction materials—their structures and functions are restricted by the chemically similar monomer building blocks they're composed of.
Now, Scripps Research chemists and additional collaborators have developed a new reaction to create unique monomers in a controlled way. This reaction, which uses nickel as a catalyst, ultimately enables scientists to create polymers with unique and modifiable properties for drug delivery, energy storage, microelectronics and more. The study was published in Nature Synthesis on August 8, 2024.
"This study shows how earth-abundant metal catalysts can unlock the path toward previously unknown materials with unparalleled structural and functional diversity," says senior author Keary Engle, Ph.D., a professor in the Department of Chemistry and dean of Graduate and Postdoctoral Studies at Scripps Research.
The Engle lab at Scripps Research focuses on developing new chemical reactions to build a wide and diverse array of small molecules, typically with applications in drug discovery. In this study, the Scripps Research team collaborated with polymer researchers at the Georgia Institute of Technology and the University of Pittsburgh to test whether their methods could be scaled up to create unique polymers.
"The properties of polymers are very much dependent on what type of chemistry is on the backbone, so if you can modify the chemistry of the building blocks, then you can easily apply it to the macromolecular structure that you're building," says Anne Ravn, Ph.D., a postdoctoral researcher in the Engle lab at Scripps Research and co-first author on the paper.
"With this project we wanted to test whether our strategy for developing small molecules could be applied to a bigger picture to provide new building blocks for polymer synthesis."
The paper's other first authors are Van Tran, Ph.D., who worked on the project as a graduate student in the Engle lab; Camille Rubel, a current graduate student in the Engle lab; Mizhi Xu, Ph.D., a former graduate student in the Gutekunst lab at the Georgia Institute of Technology; and Yue Fu, Ph.D., a former graduate student in the Liu lab at the University of Pittsburgh.
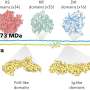
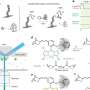
To create the new monomers, the Scripps Research team developed a chemical reaction that alters the structure of a starting molecule by using nickel as a catalyst. The nickel-catalyzed reaction added two new "functional groups" to the molecule—small side chains that confer different chemical and physical properties on the ensuing polymer, for example, how flexible, elastic or soluble it is.
Then, the team's collaborators at Georgia Institute of Technology used another chemical reaction to link the monomers together via polymerization, resulting in polymers with a unique structure.
"Most commercial polymers have two carbons in between each functional group that are not decorated with any side chains, but in this case, the functional groups are much closer in space, which creates a material with different properties," says Ravn.
In the future, the team plans to explore the impact of substituting different functional groups onto the monomers.
"Our strategy allows us to 'decorate' the molecule with much more flexibility than other methods, which gives us more freedom to explore different types of functionalities in the building blocks," says Ravn. "We're now working to expand the method to explore how introducing other types of functional groups changes the properties of the material."
Because nickel is more abundant than many other metal catalysts used in this type of chemical reaction, the researchers say that their method holds promise as an environmentally sustainable method for polymer production. They're also exploring ways to make the products even more sustainable.
"From an environmental perspective, we want to find a method to degrade these long polymers so that we can get back to the original building blocks, which would allow us to reuse them," says Ravn. "This is a tool that we hope to fine-tune in the future to ultimately create new technologies that are useful for society."
More information: Van T. Tran et al, Ni-catalysed dicarbofunctionalization for the synthesis of sequence-encoded cyclooctene monomers, Nature Synthesis (2024). DOI: 10.1038/s44160-024-00618-1
Journal information: Nature Synthesis
Provided by The Scripps Research Institute