New nickel catalyst operates in water to transform greenhouse gas into chemical feedstock
Turning plentiful carbon dioxide into a chemical feedstock would wring value from the greenhouse gas. However, the traditional approach is costly and produces unwanted byproducts. Now, scientists at Pacific Northwest National Laboratory, led by Dr. John Linehan and Dr. Aaron Appel, designed a water-soluble catalyst for this transformation. They did so by understanding each of the steps in converting carbon dioxide to formate. They delved into the expected impact of changing the reaction conditions from a non-environmentally friendly solvent to water. Changing the conditions made the reaction favorable from an energy standpoint.
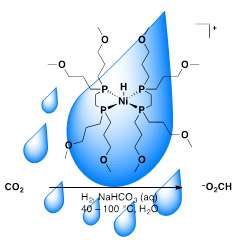
A potent greenhouse gas, carbon dioxide is abundant and readily available. It could be used as a carbon feedstock or chemical fuel precursor. The team's catalyst converts carbon dioxide to formate. This catalyst system has three distinct advantages over traditional processes. First, it uses water, which is a green solvent. Second, the catalyst uses nickel, far more available than platinum and other rare metals. Finally, it uses sodium bicarbonate as a base, rather than chemicals that are more expensive.
Numerous examples of precious transition metal catalysts for the transformation of carbon dioxide to a carbon feedstock or chemical fuel have been reported. The majority of these systems operates in organic solvents and utilizes large amounts of organic bases, which create waste and cost issues. The scientists designed, synthesized and tested a water-soluble nickel catalyst. When heated in aqueous sodium bicarbonate in the presence of high pressures of hydrogen and carbon dioxide, it yields formate. Under optimized conditions, ~270 turnovers (mole of formate per moles of catalyst) were obtained at 80 °C in 3.7 hours. The scientists determined experimentally that the slow catalytic rate is because the transfer of a hydride from the nickel complex to carbon dioxide is only slightly favorable (1 kcal/mol). Knowing that the reaction works in water and uses less expensive resources opens the doors for creating faster, more efficient first-row transition metal catalysts for carbon dioxide conversion.
More information: Samantha A. Burgess et al. Hydrogenation of CO2 in Water Using a Bis(diphosphine) Ni–H Complex, ACS Catalysis (2017). DOI: 10.1021/acscatal.7b00350
Journal information: ACS Catalysis
Provided by Pacific Northwest National Laboratory