Characterized drugs show unexpected effects
When Alexander Flemming discovered a mold on a culture plate overgrown with bacteria in 1928, he did not expect to find one of the most widely used active substances: penicillin. Accidental discoveries and the identification of active ingredients from traditional remedies, such as the morphine of the opium poppy, have shaped the discovery of new medicines for a long time.
Modern drug discovery—from chance to system
Meanwhile, major developments in chemistry and molecular biology have been made that enable a systematic and targeted search for potential active substances in modern drug discovery. First, advances in the field of organic and especially combinatorial chemistry made it possible to produce huge substance libraries and test them for their pharmacological effect in high-throughput tests. Following technological advances like the sequencing of the human genome and the development of new methods in molecular biology enabled the identification of disease-related cellular processes and their molecular key players. This paved the way towards modern drug discovery, where large libraries of molecules are screened in a high-throughput manner for their influence on relevant target molecules, mostly proteins. Identified substances, so-called hits, are optimized in their chemical structure to lead structures that are already effective in small doses and are well absorbed and distributed in the body.
No effect without side effects
This target-based drug development is very successful in identifying new drug candidates that prevent the target proteins from functioning or interacting with other proteins. However, potential drug candidates are rarely specific and very often also act on related proteins that have a similar function or structure. "It is not uncommon that an initially promising drug candidate unexpectedly shows serious side effects in a later phase of its longstanding development, thus limiting or even preventing its clinical use," says Slava Ziegler.
In search of unknown bioactivities
In order to track down possible side effects during drug development, potential drug candidates are screened in assays for their effect on known protein classes, biological processes and certain cellular properties. However, these tests can only reflect the expected bioactivity since the number of known target molecules in the cell is limited. So-called profiling approaches now offer the possibility of detecting a wider spectrum of activity. These unbiased tests investigate the influence on hundreds of cellular or genetic parameters recorded in a substance's profile that is compared with profiles of reference substances with known effects.
When drug profiles match
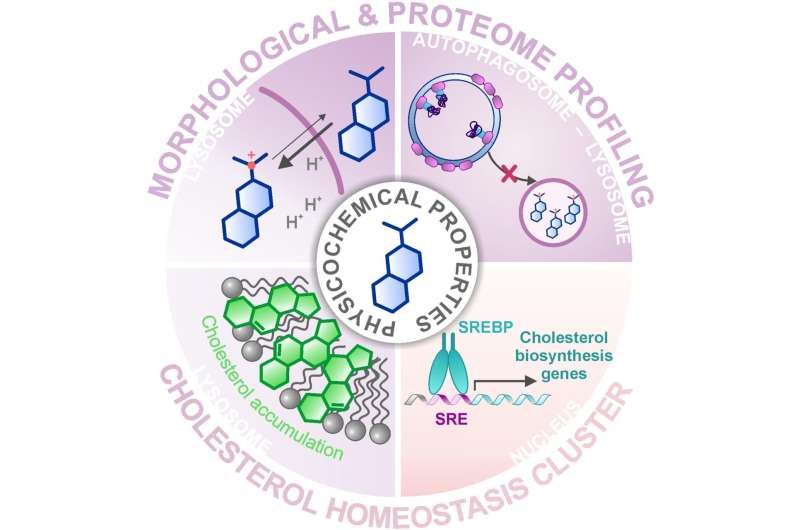
In their latest study, the group of Herbert Waldmann and Slava Ziegler combined two of these profiling approaches to identify bioactive substances from a library of about 15000 natural product-inspired molecules and compared them with the profiles of known, active compounds. Applying the Cell Painting Assay, where functional areas of the cell are stained and then microscopically examined for changes, a large cluster of substances with similar profiles was identified. However, it was not possible to predict the mode of action of the cluster since the associated reference compounds had various activities or target molecules. With a subsequent search using proteome profiling, in which the quantities and thus the regulation of thousands of proteins was examined, the researchers were able to narrow down the cluster to a common activity—the modulation of cholesterol homeostasis—an unexpected biological activity for the most reference substances in the cluster.
Two birds with one stone: Identifying new bioactivities and side effects
But how can substances with very different target molecules trigger the same effect? The researchers revealed that most of the compounds in the cluster accumulate in the lysosome, an organelle where cholesterol is stored temporarily for its further function in the cell. The lysosome has a lower pH value than the rest of the cell, and this is crucial for the functioning of the lysosomal digestive enzymes that process foreign and the cell's own biomolecules. In the lysosome, the substances from the cluster described increase the pH value and thus disrupt the function of this organelle and, in particular, the cholesterol balance of the cell. The fact that the compounds accumulate in the lysosome is not due to a specific target molecule in the lysosome but to their chemical and physical properties, which they have obtained through their structural optimization for improved solubility.
"Interestingly, disturbed cholesterol balance has already been linked to some drugs on the market, such as antipsychotics" notes Tabea Schneidewind, first author of the study. " With the combination of the two search strategies, we can kill two birds with one stone: unveil unknown side effects and identify new active substances and modes of action" says Slava Ziegler.
Targeting cholesterol homeostasis could possibly also disturb SARS-CoV-2 infections
Influencing cholesterol homeostasis seems to be a common feature of many compounds and should be taken into account when evaluating side effects of active substances. However, the observed activity is not per se undesired. Currently, drugs and compounds with known modes of action are being intensively studied for inhibition of SARS-CoV-2 infection of host cells, and many compounds of our cluster have been identified to suppress this process. Interestingly, membrane cholesterol and thus proper cholesterol homeostasis are crucial for Sars-CoV-2 infection as shown in several studies. Our data most likely explain the reason for the activity of these compounds against the virus: they alter cholesterol biosynthesis and localization in cells, which impairs Corona-Virus infection", says Slava Ziegler.
More information: Tabea Schneidewind et al, Combined morphological and proteome profiling reveals target-independent impairment of cholesterol homeostasis, Cell Chemical Biology (2021). DOI: 10.1016/j.chembiol.2021.06.003
Journal information: Cell Chemical Biology
Provided by Max Planck Society