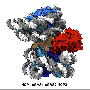How proteins bind 'hidden' DNA

How can proteins bind DNA in the cell nucleus, where it is present in form of chromatin, tightly wrapped around histones and therefore mostly inaccessible? Recently, several studies began to uncover the various strategies used by DNA-binding proteins to solve this problem. In a Cell "Leading Edge review," Alicia Michael and Nico Thomä look at these findings and highlight general principles that aim to help predict how a protein recognizes a specific stretch of DNA, even when "hidden" in chromatin.
Nearly 35 years ago, scientists determined for the first time the structure of a protein bound to DNA, contributing to a good understanding of how proteins bind a specific stretch of DNA. However, most studies since then have been conducted on isolated DNA in a test tube, whereas DNA in the cell is wrapped around histone proteins that form nucleosomes, the elementary building blocks of chromatin. When a DNA sequence is close to histones, it becomes inaccessible to other proteins such as transcription factors (TFs) or repair enzymes. For this reason, DNA-binding proteins need different strategies to interact with DNA when it is packaged in the chromatin fiber.
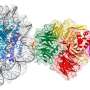
The Thomä group is studying the interaction of proteins with DNA in the context of chromatin from a structural perspective. Several structures of proteins binding to DNA on nucleosome have recently been solved by a number of laboratories, including the Thomä and Schübeler groups at the FMI, as well as by the Cramer, Taipale and Kurumizaka laboratories. These structural insights were made possible by cryo-electron microscopy (cryo-EM) technology that allows visualization of complex assemblies at the molecular level. Intrigued by these recent insights, Alicia Michael, a postdoc in the Thomä lab, and Nicolas Thomä discuss the interplay between DNA-binding proteins with specific DNA sequences or modifications on nucleosomes in a review article.
"A key conclusion from these findings is that the position of the nucleosome and that of the DNA binding site really matters," says Michael. "In the past we thought that nucleosomes were just amorphous obstacles for DNA binding proteins. But now that we are able to precisely look at the molecular details, we can tell that it's a lot more complex." Michael explains that the strategy used by a protein to bind a sequence on DNA depends on several factors, most importantly the position of the DNA binding site on the nucleosome and the DNA binding fold (the part of the protein that binds to DNA). "Nucleosomes can selectively give access to a binding site and let a transcription factor or a repair protein bind. As such, they are ideally suited to gate access for gene regulation and DNA repair."
Many questions remain − for example to what extent DNA binding proteins require assistance from other proteins such as "remodelers" that are able to put nucleosomes in certain positions throughout the genome. However, "the progress made in the field over the past few years has been phenomenal," says Thomä. "We didn't expect the rules for how proteins bind DNA on nucleosomes to be that different compared to what we know about proteins binding isolated DNA. The structures and analysis further our understanding of the fundamental principles of how eukaryotes—from yeast to humans—regulate and repair their genome."
More information: Alicia K. Michael et al, Reading the chromatinized genome, Cell (2021). DOI: 10.1016/j.cell.2021.05.029
Journal information: Cell