May 28, 2020 feature
Topology control of human fibroblast cells monolayer by liquid crystal elastomer
Eukaryotic cells within living tissues can affect important physiological processes such as apoptosis and cell migration based on dynamic pattern formation with spatially varying orientations. However, it is yet challenging to project a predesigned map of orientational order onto a growing tissue in the lab. In a new study now published on Science Advances, Taras Turiv and a research team in chemical physics, advanced materials and biomedical sciences at the Kent State University, Ohio, U.S., detailed a new approach to produce cell monolayers of human dermal fibroblasts. They predesigned the orientation patterns and topological defects using a photoaligned liquid crystal elastomer (LCE) that swelled anisotropically in an aqueous medium. The team inscribed the patterns into the LCE, and the tissue monolayer replicated the patterns to cause strong variations to cell phenotypes (size and shape), their surface density and number density fluctuations. The new approach can control the collective behavior of cells in living tissues during cell differentiation and tissue morphogenesis for broad applications in bioengineering and regenerative medicine.
Cells that constitute living tissues often exhibit orientational order when in close contact due to mutual alignment of anisometric cells. The direction of average orientation can vary in space and time to produce topological defects known as disclinations. Such defects can move within the tissue to play an important role during compressive-dilative stresses and processes, including dead cell extraction. The ability to design a tissue scaffold of living cells with orientational order and control is important for biomedical researchers so as to investigate and manipulate living matter. Scientists have already produced ordered cell assemblies at lithographically fabricated surfaces, including the edges of microchannels, in microgrooves and surfaces with material stiffness gradients. In this work, Turiv et al. designed tissues with a high degree of orientational order and predetermined spatially varying direction, based on a template of director patterns on LCE substrates. The team used human dermal fibroblast (HDF) cells as the building units of the templated tissue.
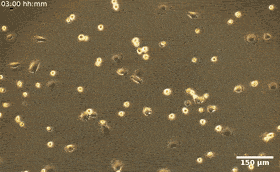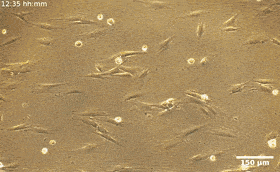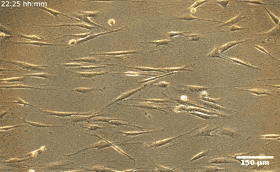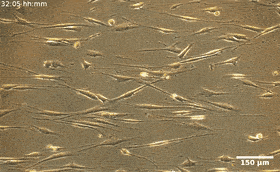
Fibroblasts are the most common mammalian connective tissue cells and they usually maintain a flat elongated shape with important roles during tissue repair and restructure, as well as wound healing. Scientists can reprogram these cells into pluripotent stem cells for promising applications in diagnostics and therapy. In this work, the combined effects of cell seeding and division of patterned HDF tissues on predesigned LCE substrates produced confluent tissues. The structured LCE had a marked impact on the tissue, where they controlled the alignment pattern and spatial distribution of cells, their density, fluctuations, and phenotype. The patterned LCE showed locations of topological defects in tissues through anisotropic surface interactions at predetermined locations. Since the cellular alignment and topological defects can control biochemical processes at the microscale, this work opens the possibility of engineering surfaces for controlled tissue patterning in order to design them for specific functions.
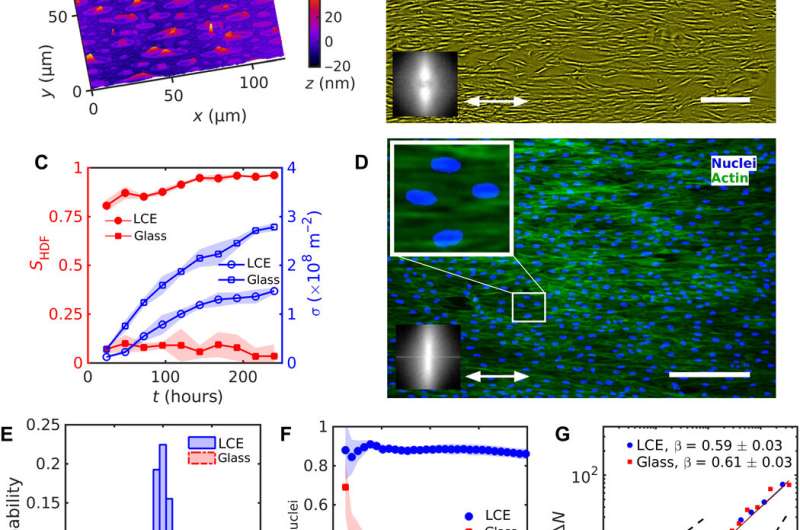
During the experiments, Turiv et al. supported the LCE substrate by a glass plate and covered it with indium tin oxide (ITO) to reduce surface roughness, followed by coating a layer of photosensitive azo dye and ultimately covered the substrate with an aqueous medium of cell culture. The surface grains on the material served as a guiding rail for HDF cells. When the HDF cells were suspended in cell culture, they appeared round but after setting into the substrate, they developed an elongated appearance. The scientists recorded confluence (growth) results from combined effects of cell seeding. The results showed that the orientational order occurred due to direct interactions between cells and the LCE substrate. The substrates helped align both bodies and nuclei of HDF cells as an important feature for many cell functions including protein expression, motility, metabolism and differentiation.
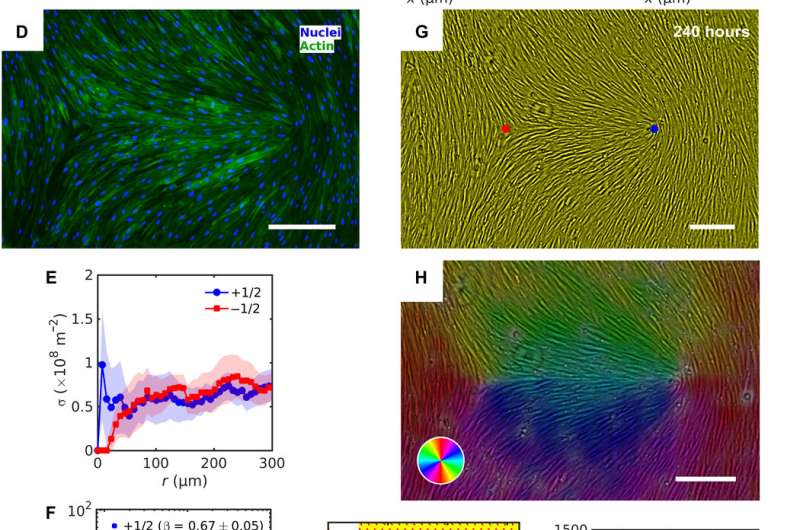
The HDF cells on LCE self-organized into aligned assemblies following pre-imposed directions. The team noted the behavior of cells and cell density to vary as they approached defect cores and other topological inconsistencies (bent type defects or splay type defects) on the LCE substrates. The substrates markedly impacted the HDF cells that were in contact with each other, resulting in collectively strong differences in the size and shape of cells. The marked differences indicated the influence of the predesigned patterns on the HDF cell phenotype (size and shape). Based on additional results, Turiv et al. credited the number density fluctuations in tissues to be influenced by the surface charge of director patterns and studied the issue in detail in a larger surface area.
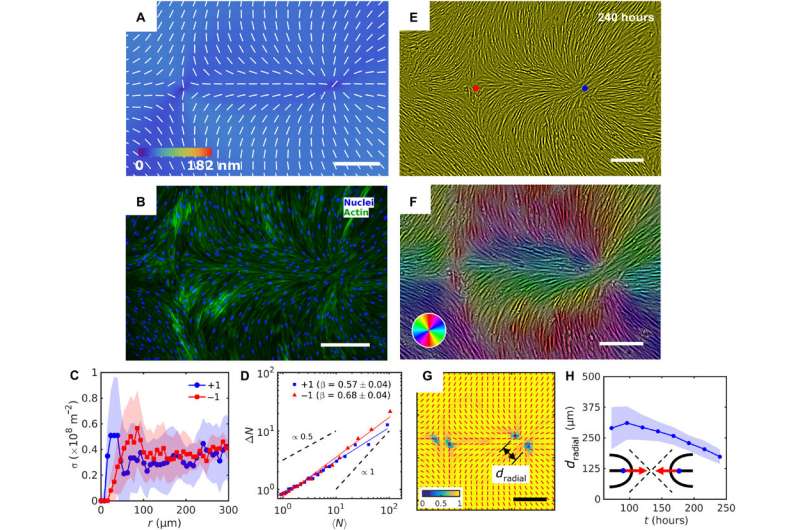
In this way, Taras Turiv and colleagues showed the dynamics and propagation of defects in patterned tissues and how they could be halted through surface anchoring forces. The scientists used LCE substrates with photopatterned structures of varying molecular orientations to grow biological tissues with predesigned cell alignment. The substrates affected cell alignment as well as cell surface density and cell phenotypes. The team noted higher density of cells in defect cores with positive topological charge, while cell density was lower near negative defects. The cells mechanistically aligned to the substrates by swelling upon contact with the aqueous cell culture medium, followed by aligning to predesigned photopatterned direction. This approach will allow materials scientists and bioengineers to design biological tissues with predetermined cell alignment and precise location of orientational defects. The outcomes can facilitate controlled cell migration, differentiation. and apoptosis. The work can be further optimized to advance the understanding of fundamental mechanisms underlying tissue development and regeneration.

More information: Taras Turiv et al. Topology control of human fibroblast cells monolayer by liquid crystal elastomer, Science Advances (2020). DOI: 10.1126/sciadv.aaz6485
Guillaume Duclos et al. Topological defects in confined populations of spindle-shaped cells, Nature Physics (2016). DOI: 10.1038/nphys3876
D.-H. Kim et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs, Proceedings of the National Academy of Sciences (2009). DOI: 10.1073/pnas.0906504107
Journal information: Science Advances , Nature Physics , Proceedings of the National Academy of Sciences
© 2020 Science X Network