Single-molecule imaging reveals how myosin moves to bring about muscle contraction

In a research first, molecular biologists at RIKEN have directly visualized the motion of a critical motor protein at the single molecular level. This achievement could aid in the hunt for new ways to treat diseases associated with myosin malfunction.
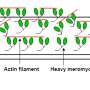
Muscles contract by sliding between thick filaments of myosin and thin filaments of actin. But there has been a longstanding controversy about the dynamics of this molecular machine. Researchers have been debating whether myosin travels by ratcheting along actin or simply by marching progressively forward in a series of power strokes.
To resolve this question, a team led by Mitsuhiro Iwaki of the RIKEN Center for Biosystems Dynamics Research watched individual myosin molecules in action. They did this by assembling strands of DNA into bundles of rope-like fibers, creating rods that resembled the thick filaments found in muscle tissue. The researchers then attached myosin proteins along the DNA structures in precise positions (Fig. 1), and, using advanced imaging techniques, snapped atomic-scale pictures of myosin interacting with its partner protein actin under biologically realistic geometric conditions.
The team showed with microsecond resolution that the head of myosin first binds weakly to actin. That bond strengthens, however, as myosin moves along the actin filament and finds the position for maximal force generation. Once in place, the lever arm below the myosin head swings in a two-step fashion, eliciting an oar-like stroke that powers muscle contraction. That motion is flexible and reversible, though, allowing multiple myosins to work cooperatively and in unison for efficient muscle function.

This demonstration of how myosin moves to bring about muscle contraction reveals that both proposed mechanisms, namely the ratchet one and the power-stroke one, occur in tandem.
According to Iwaki, his team's state-of-the-art visualization technique—which takes advantage of 3-D DNA origami and high-speed atomic force microscopy—now offers a platform for scientists to better understand why mutant forms of myosin can cause forms of heart failure in which the cardiac muscle thickens, making it hard for the organ to pump blood. That kind of information should help pharmaceutical companies in the search for drug compounds that can coax malfunctioning myosin in the heart to do its job properly.
"Because our novel assay system can visualize the basic mechanical processes of myosin, we can precisely examine the effect of drug candidates on myosin function," Iwaki says. "We can thus contribute to advancing precision medicine approaches to heart disease."
More information: Keisuke Fujita et al. Direct visualization of human myosin II force generation using DNA origami-based thick filaments, Communications Biology (2019). DOI: 10.1038/s42003-019-0683-0
Journal information: Communications Biology
Provided by RIKEN