Novel intermolecular surface force reveals actomyosin driving mechanism
The actin and myosin complex (actomyosin) generates contraction force of a muscle utilizing the adenosine triphosphate (ATP) hydrolysis reaction. Many attempts have thus been made to explain the molecular origin of the actomyosin motility.
A myosin power stroke model, proposed by Huxley and Simmons in 1971, initiated much research including atomic-structure studies and the investigation of the molecular biology of myosin and actin molecules. The power stroke model, modified lately, is widely adopted in standard biology textbooks.
However, there remains a serious problem. According to the experimental thermodynamics data, the ATP hydrolysis in the myosin head does not produce a myosin state with high enough energy to generate the contraction force.
Now, a research group, led by Emeritus Professor Makoto Suzuki at Tohoku University in collaboration with Professor Nobuyuki Matubayasi at Osaka University, has succeeded in explaining the actomyosin driving mechanism according to the experimental thermodynamics data.
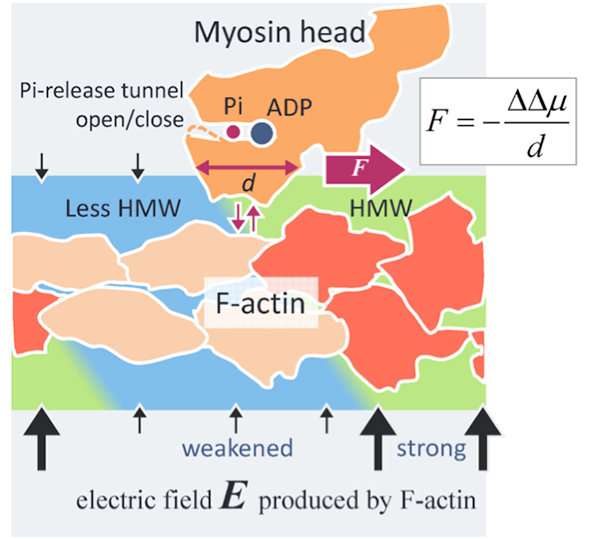
In the study, the water structure in close proximity of an actin filament (F-actin) is modified upon binding with a myosin head hydrolyzing ATP to F-actin, that leads to a change in the affinity to the myosin head and thus to the generation of the driving force of actomyosin.
The presence of the novel intermolecular surface force - which was demonstrated for the first time based on the present hydration analyses - was a marked discovery. This article is, therefore, the first to successfully unveil the actomyosin driving mechanism by introducing a novel intermolecular surface force.
More information: Makoto Suzuki et al, Physical driving force of actomyosin motility based on the hydration effect, Cytoskeleton (2017). DOI: 10.1002/cm.21417
Provided by Tohoku University