Protective protein in the eye lens affects protein oxidation
The lens of the human eye comprises a highly concentrated protein solution, which lends the lens its great refractive power. Protective proteins prevent these proteins from clumping together throughout a lifetime. A team of scientists from the Technical University of Munich (TUM) has now uncovered the precise structure of the alpha-A-crystallin protein and, in the process, discovered an important additional function.
The refractive power of the human eye lens stems from a highly concentrated protein solution. These proteins are created during embryonic development and must then function for a whole life, as the lens has no machinery to synthesize or degrade proteins.
When lens proteins are damaged, the result is cataract—a clouding of the lens—or presbyopia. This is where protective proteins come in: They ensure that the proteins of the eye retain their form even under adverse environmental influences.
"The two protective proteins alpha-A and alpha-B-crystallin make up around 30 percent of the proteins in the human eye and are extremely important for the function of the lens," says Christoph Kaiser, first author of the publication in the journal Nature Structural and Molecular Biology.
Structure of a multifaceted protein
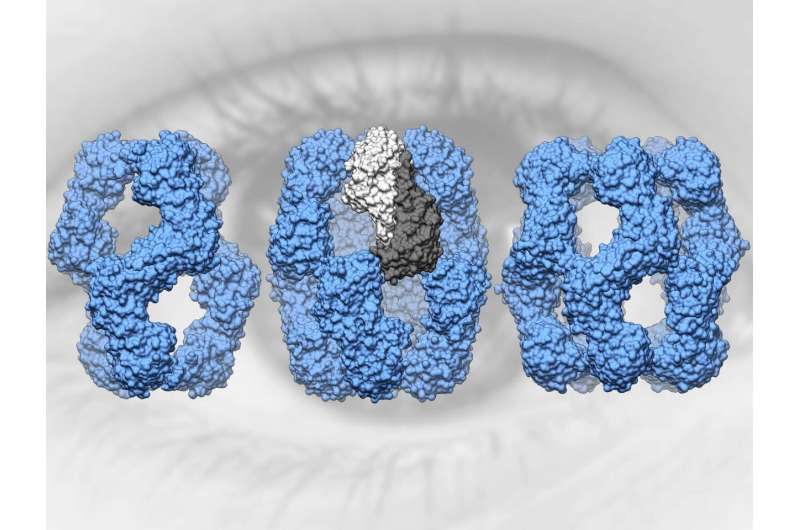
Attempts to determine the structure of alpha-A-crystallin were unsuccessful for over 40 years. The breakthrough came for a research team led by the TUM professors Sevil Weinkauf, professor of electron microscopy and Johannes Buchner, professor of biotechnology, by combining cryo-electron microscopy, mass spectrometry, NMR spectroscopy and molecular modeling.
"Alpha-A-crystallin is extremely multifaceted," says Sevil Weinkauf. "This makes it very difficult to determine its structure. It was only after developing a new strategy for data analysis that we were able to demonstrate that in solution it takes on different structures with 12, 16 or 20 subunits."
Protection against oxidation
The typical function of protective proteins is to help other proteins maintain their form when stressed, by high temperatures, for example. This is why they are also referred to as chaperones.
Alpha-A and alpha-B-crystallin, too, have this function. In addition, human alpha-A-crystallin has two cysteine residues. The sulfur atoms in these residues can form disulfide bridges. In-depth biochemical studies have shown that this bridging has a significant impact on various properties of the protein molecule.

"A common theory is that the disulfide bridges result from damage to the protein, for example through oxygen," says Johannes Buchner. "Our results suggest that alpha-A-crystallin might play an active role in protecting other proteins from oxidation."
Motivation for further research
The research team's investigations demonstrate that oxidized alpha-A-crystallin can even transfer the existing disulfide bridge to other proteins. "This ability corresponds to that of a protein disulfide oxidase," says Christoph Kaiser. "Alpha-A-crystallin can thus influence the redox state of other lens proteins. This function also explains why roughly half of the alpha-A-crystallins in embryos already have such disulfide bridges."
"Around 35 percent of all cases of blindness can be attributed to cataracts, says Sevil Weinkauf. "The molecular understanding of the functions of eye lens proteins forms an essential basis for developing prevention and therapy strategies. The realization that alpha-A-crystallin also plays an important role in protecting against oxidation will now spawn further research."
More information: Christoph J. O. Kaiser et al, The structure and oxidation of the eye lens chaperone αA-crystallin, Nature Structural & Molecular Biology (2019). DOI: 10.1038/s41594-019-0332-9
Journal information: Nature Structural and Molecular Biology , Nature Structural & Molecular Biology
Provided by Technical University Munich