New field of application for versatile helper
In Alzheimer's disease proteins clump together to long fibrils causing the death of nerve cells. Small heat shock proteins can counteract this effect. Scientists, therefore, hope to deploy them as agents in the treatment of neurodegenerative diseases. Using the example of a small heat shock protein, researchers at the Technical University of Munich (TUM) and the Helmholtz Zentrum München have now uncovered how the protein interacts with other proteins.
Small heat shock proteins are the "catastrophe aid workers" of the cell. When exposed to strong heat or radiation, vital cell proteins lose their structure and clot up to entangled clumps. Once these clumps have formed there is no return – the proteins become useless and the cells begin to die. Small heat shock proteins, however, attach to the deformed proteins before they clump together and preserve them in a soluble state – helping to restore their proper form.
Promising candidate for new forms of therapy
The helper proteins are true multi-talents. They can bind large numbers of badly folded proteins and keep them from clumping together. This also includes the potentially disease-causing proteins that collect in the cells of patients with neurodegenerative disorders – for example, beta amyloids that agglomerate to form long fibrils in the nerve cells of Alzheimer's patients. Heat shock proteins are also associated with other nervous system disorders like Parkinson's disease and multiple sclerosis.
Although it is still unclear what role these catastrophe aid workers play in the various ailments, they are already being considered as models for agents in new medications. If the precise mechanisms by which these heat shock proteins hook up to their disease-causing counterparts were known, scientists could deploy this knowledge to develop agents utilizing these mechanisms to fight disease.
Two ways out of the chaos
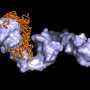
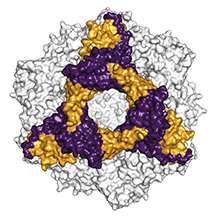
A group of researchers led by Bernd Reif, professor at the Department of Chemistry of the Technical University of Munich (TUM) and group leader at the Helmholtz Zentrum München, have now succeeded in uncovering precisely this mechanism. Using a refined procedure of solid-state nuclear magnetic resonance spectroscopy (solid-state NMR), they managed, for the first time ever, to identify the sites in the alpha-B-crystallin that attach to the beta-amyloid.
It is the first direct structure analysis of a complete heat shock protein during interaction with a bonding partner, because the catastrophe aid workers do not make the observer's job easy. "Alpha-B-crystallin exists in various different forms comprising 24, 28 or 32 subunits that are permanently being swapped," explains Reif. "In addition, it has a large molecular weight. These factors make structure analysis very difficult."
In close collaboration with his TUM colleagues Johannes Buchner, professor of biotechnology and Sevil Weinkauf, professor of electron microscopy, Reif determined that the small heat shock protein uses a specific non-polar beta-sheet structure pile in its center for interactions with the beta-amyloid, allowing it to access the aggregation process in two locations at once: For one it attaches to individual dissolved beta-amyloids, preventing them from forming fibrils. In addition, it "seals" existing fibrils, so that further amyloids can no longer accumulate.
Template for artificial protein construction
Precise knowledge about the way in which alpha-B-crystallin attaches to the Alzheimer's protein is particularly interesting for researchers using so-called "protein engineering" to develop new agents that bind specifically to beta-amyloid and similar proteins. If the newly discovered beta-sheet structure idea could be integrated as building blocks into such artificially designed proteins, it would improve their ability to attach to the disease-causing fibrils – a first step in the development of new agents against Alzheimer's and other neurodegenerative diseases.
In future work, the scientists want to take a closer look at the N-terminal region of the alpha-B-crystallin. As Reif and his colleagues have discovered, it binds protein types that, unlike the beta-amyloid, clump together in an unordered manner. The researchers will be supported by the new NMR Center that is currently under construction at the Garching campus of the Technical University of Munich and slated to open in 2017. A further facility geared specifically to solid-state NMR is currently under construction at the Helmholtz Zentrum in Neuherberg.
More information: Andi Mainz et al. "The chaperone αB-crystallin uses different interfaces to capture an amorphous and an amyloid client," Nature Structural & Molecular Biology (2015). DOI: 10.1038/nsmb.3108
Journal information: Nature Structural & Molecular Biology
Provided by Helmholtz Association of German Research Centres